Signalment:
Gross Description:
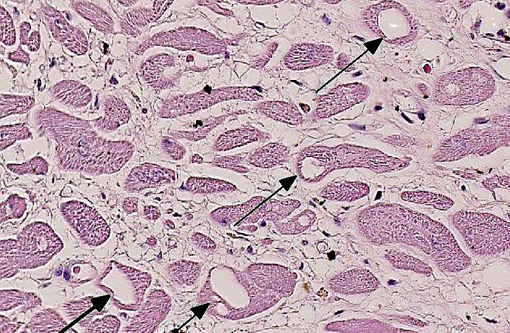
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Toxicity is related to the total cumulative dose administered, as well as to the acute peak concentration levels of the compound. Increased risk of DOX cardiotoxicity has also been correlated with young age, concurrent administration of additional chemotherapy compounds and viral diseases. Retrospective studies of human patients have revealed that more than 4% of patients who receive a cumulative dose of 500-550 mg per square meter of surface area develop congestive heart failure. The incidence rises to more than 18% over 551-600 mg/m².(8) Based on several clinical studies limiting the cumulative dose to less than 450 mg/m2 is the best line of defense against DOX cardiotoxicity. Alternative methods have been the concurrent administration of antioxidants, iron chelators, use of DOX analogues. However, none of the approaches have had major success. Recently, combined administration of the hematopoietic cytokines EPO, G-CSF and TPO has prevented DOX cardiomyopathy in animal models.(8) In this dog, a standard chemotherapy protocol for canine hemangiosarcoma with a cumulative dose of DOX lower than 240 mg/m² (recommended to minimize chronic cardiac toxicity) was used.(3) Myocardial vacuolization is described in early and chronic toxicity with a wide range of and from 122 to 265 mg/ m² body surface area (BSA) in clinical cases.(3) The overall cumulative dose causing fatal cardiomyopathy is therefore controversial, and dogs are generally considered more sensitive to cardiotoxic effects of doxorubicin than humans, where cardiomyopathy and congestive heart failure are reported to occur at a total dose greater than 550mg/ m² BSA.(2)
Prognosisis very poor in human patients that develop cardiomyopathy within four weeks after administration of DOX and the majority die within two weeks after onset of symptoms.(8) Among long survivors after DOX therapy, several develop heart failure six to ten years after conclusion of chemotherapy. The late-onset cardiotoxic effects of conventional anthracycline therapy highlight the need of lifelong monitoring the cardiac status in human patients. Several techniques (i.e. radionuclide angiography assessment of left ventricular ejection fraction, electrocardiography and echocardiographically derived ejection fraction) have been proven to be poor indicators of early changes and subclinical myocardial injury.(3,8) Serial endomyocardial biopsies are currently considered the most sensitive and specific indicators of doxorubicin-induced injury.(8) Biopsies are examined by electron microscopy and graded applying a semiquantitative scoring system(1) based on the percentage of myocytes affected by myofibrillar loss and cytoplasmic vacuolization. Ultramicroscopic markers including myofibril loss, retention of sarcoplasmic reticulum and cytoplasmic vacuolation are utilized to grade injury on a scale of 1 to 3; biopsy samples in which fewer than 5% of cells have typical changes are given a grade 1 while those with changes over 36% are graded 3, the highest severity.(8) This grading shows a linear correlation with left ventricular function determined by radionuclide angiocardiography and is helpful for clinical determination of continuation of DOX therapy.(8)
In veterinary medicine, no sensitive predictor tests are currently available to monitor patients treated with doxorubicin. Herman and co-workers (1981) observed that plasma enzymes CPK (creatine phosphokinase), LDH (Lactate Dehydrogenase) and SGOT (Serum Glutamic Oxaloacetic Transaminase) were not reliable indicators of slowly progressive cardiac damage. Similarly to human patients, sequential echocardiograms and EKGs (electrocardiogram) are not considered sensitive predictors for canine cardiomyopathy since no consistent correlation between the severity of rhythm disturbance and the pathologic myocardial changes have been observed.(2) Myocardial biopsies in dogs, although not routinely performed due to their invasiveness, have been experimentally proven to be a sensitive test to monitor the early doxorubicin-associated cardiotoxicity.(7)
Because DOX cardiotoxicity is dose dependent, it has been used to experimentally induce heart failure in different animal species such as dog, sheep, goats and rodents.(5) DOX is delivered by intravenous and intracoronary injections at small doses to induce heart failure without systemic toxicity. Experimental DOX heart failure develops via bilateral enlargement, ventricular wall thinning with decreased cardiac output. This model has been utilized to study several treatments for cardiac failure however the model has several limitations: the degree of left ventricular dysfunction varies, is characterized by high incidence of arrhythmias, high cost of multiple intracoronary injections and the irreversible and progressive heart damage.(5)
The mechanism of action of DOX and other antracycline compounds on tumor cells are still a matter of controversy. Suggested mechanisms are: intercalation into the DNA molecule leading to inhibition of transcription, generation of reactive oxygen species leading to lipid peroxidation and DNA damage, DNA binding and alkylation, DNA cross-linking, interference with DNA unwinding and helicase activity, inhibition of topoisomerase II and induction of apoptosis.Â
Anthracycline compounds including DOX, produce reactive oxygen species (ROS) interacting with mitochondrial enzymes. ROS are produced in vitro by high concentration of DOX that binds to iron forming DOX-iron complexes that bind to DNA and induce production of partially reduced oxygen compounds. These radicals can damage DNA via strand break formation. However, high concentrations of DOX seem necessary and antioxidant compounds do not diminish DOX cytotoxicity.(8)
The mechanism of DOX-cardiomyopathy remains unclear but seems different from the one underlying DOXs anti-tumour activity. Most studies support the view that increase in oxidative stress evidenced by increases in ROS and lipid peroxidation play a key role along with reduction in antioxidant levels and sulfhydryl groups.(3,6) Other associated mechanisms proposed have been: inhibition of protein and DNA synthesis, lysosomal and mitochondrial changes, alteration of sarcolemmal Ca2+ transport, attenuation of adenylate cyclase, ATPase activities, imbalance in myocardial electrolytes and several others.(8) Also, DOX downregulates the expression of cardiac-specific genes including contractile proteins (alpha-actinin, myosin light and heavy chains, troponin I, desmin), and sarcoplasmic reticulum proteins. The reduction of myocardial contractility can be directly associated with reduction in muscle proteins. DOX additionally induces apoptosis of endothelial cells and cardiomycytes contrary to its cystostatic effect in tumor cells. This latest mechanism seems related to p53 activation. However, the role of apoptotic pathways in DOX cardiotoxicity is still controversial.(8)
Transcriptional profiling via genome wide transcriptome analysis has been utilized to determine early cardiac response to DOX in a rat model perfused with DOX leading only to mild cardiac dysfunction.(9) The main characteristics of cardiomyocyte reprogramming were the repression of transcripts involved in cardiac stress response and stress signaling, modulation of genes with cardiac remodeling capacity and upregulation of energy related pathways. This latest research supports the hypothesis that blunted response to stress and reduced danger signalling are prime components of DOX toxicity and can drive to cardiomyocyte damage.(9)
JPC Diagnosis:
Conference Comment:
References:
2. Gralla EJ, Fleischman RW, Luthra YK, Stadnicki SW. The dosing schedule dependent toxicities of adriamycin in beagle dogs and rhesus monkeys. Toxicology. 1979;13:263-73.Â
3. Mauldin GE, Fox PR, Patnaik AK, Bond BR, Money SC, Matus RE. Doxorubicin-induced cardiotoxicosis. Clinical features in 32 dogs. J Vet Inter Med. 1992;6:82-88.
4. Miller LM, Van Vleet JF, Gal A. Cardiovascular system and lymphatic vessels. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Disease. 5th ed. St Louis, MO: Elsevier Mosby; 2012:555.
5. Monnet E, Chachques JC. Animal models of heart failure: what is new? Ann Thorac Surg. 2005;79:1445-1453.
6. Ogilvie GK, Powers BE, Mallinckrodt CH, Withrow J. Surgery and doxorubicin in dogs with hemangiosarcoma. J Vet Intern Med, 1996;10:379-84.
7. Sparano BM, Gordon G, Hall C, Iatrapoulos MJ, Noble JF. Safety assessment of new anticancer compound, mitoxantrone, in Beagle dogs: comparison with doxorubicin. II. Histologic and ultrastructural pathology. Cancer Treat Rep. 1982;66:1145-1158.
8. Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330-352.
9. Tokarska-Schlattner M, Lucchinetti E, Zaugg M, Kay L, Gratia S, Guzun R, et al. Early effects of doxorubicin in perfused heart: transcriptional profiling reveals inhibition of cellular stress response genes. Am J Physiol Regul Integr Comp Physiol. 2010;298: R1075-1088.