Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 10
28 November 2018
CASE II: 9428-12 (JPC 4048660).
Signalment: Two-years-old, neutered, male, domestic shorthair cat, (Felis domesticus)
History: Patient presented to emergency clinic in lateral recumbency with oral ulcers, mild dyspnea, hypothermia, and mild nystagmus. Reportedly, the patient had been anorexic. The patient died within one hour of presentation.
Gross Pathology: Spleen was enlarged with a roughened serosal surface. Multiple white pinpoint foci were observed on the serosal surface. The cut surface bulged and had a granular appearance. Liver was swollen with rounded margins and multiple white pinpoint foci were present on the serosal and cut surfaces.
Laboratory results: Neutropenia, hyperbilirubinemia, elevated ALT. Francisella tularensis was isolated from the spleen and from a lymph node sample.
Microscopic Description:
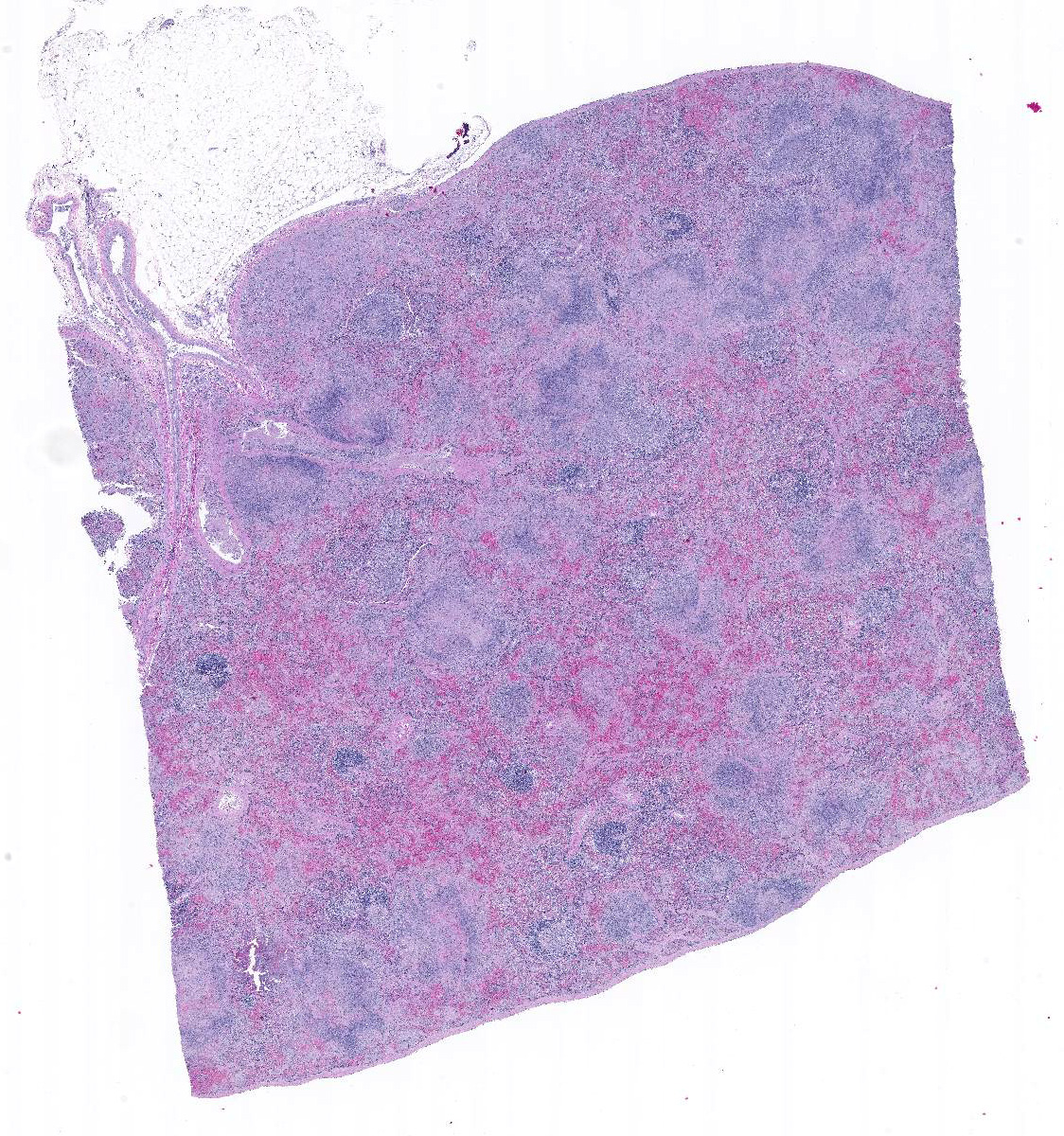
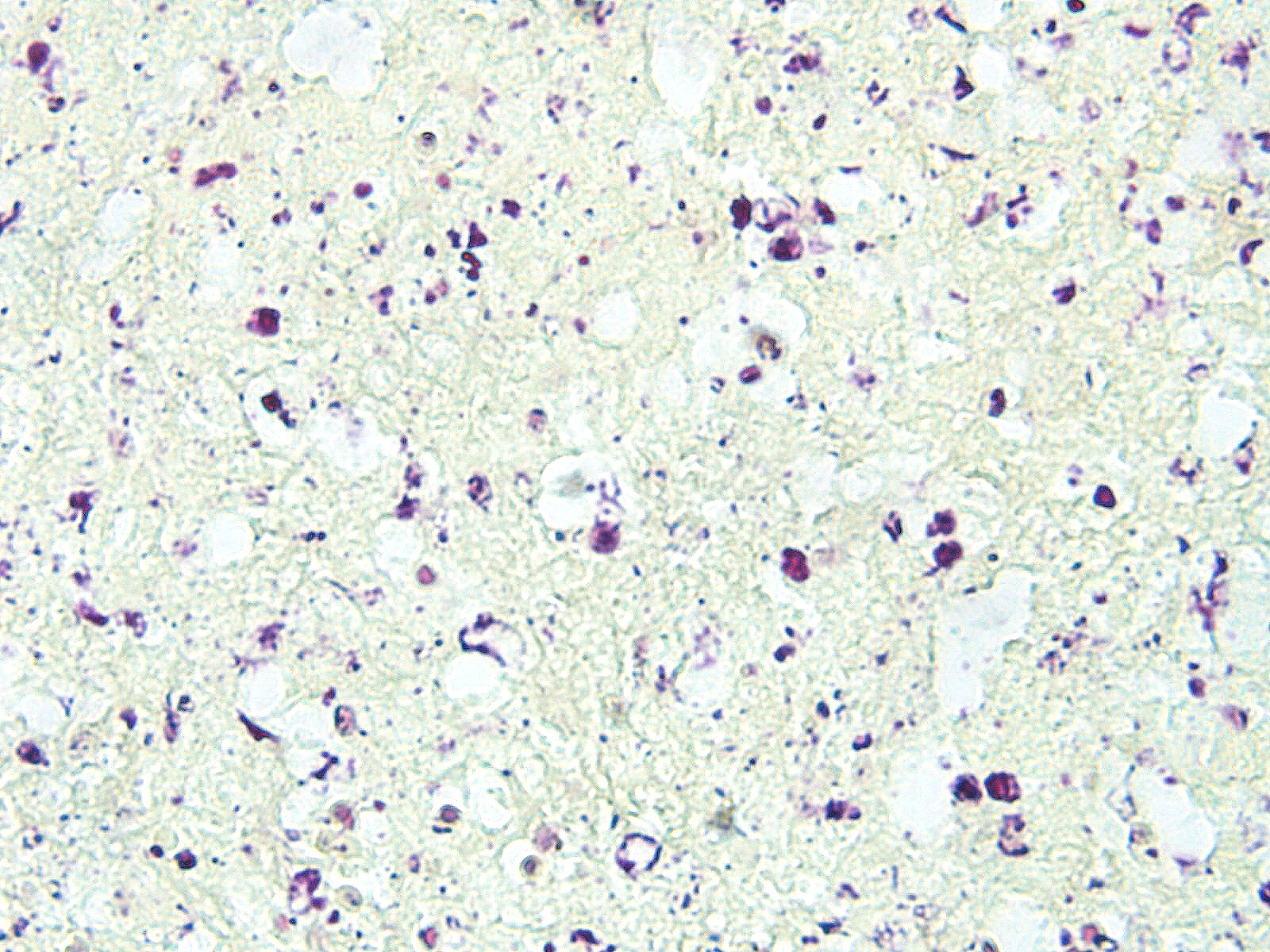
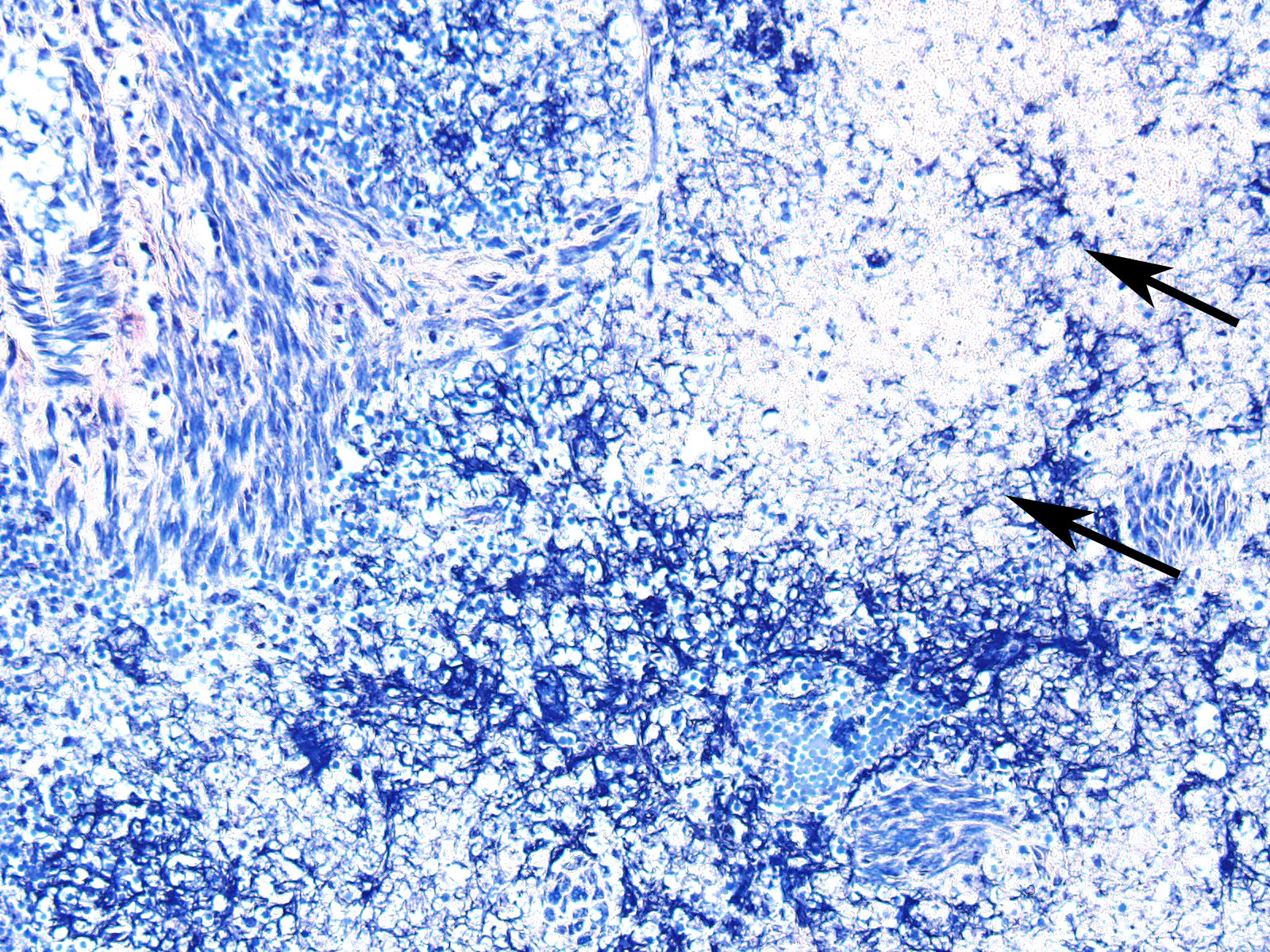
In sections of spleen, there is disseminated and coalescing necrosis of germinal centers which extends into adjacent red pulp. Foci of necrosis are accompanied by an inflammatory response comprised of neutrophils and macrophages. Scattered thrombosed blood vessels can be seen mainly associated with the capsule and near the hilus. Variably sized and shaped aggregates of homogenous eosinophilic material is present in many germinal centers. Mesothelial cells on the serosa are reactive characterized by cuboidal appearance.
Contributor’s Morphologic Diagnoses:
1. Severe, subacute, multifocal, coalescing, necrotic, splenitis.
- Splenic amyloidosis – germinal centers
Contributor’s Comment: Francisella tularensis is a gram-negative, encapsulated, facultative intracellular pathogen.7 F. tularensis is subdivided into two subtypes. Type A is F. tularensis subsp. tularensis and has and infectious dose in humans of <10 CFU’s, whereas type B is F. tularensis subsp. holarctica which as an infectious dose of <103 CFU and a milder form of tularemia in humans.7 The organism is abundant in nature and infects many mammalian and arthropod species.11 F. tularensis type A has been isolated from cats on numerous occasions and can be transmitted from cats and other animals (deer, personal experience) to humans. 5,10,13
Diagnosis, in some cases may be difficult, but culture appears to be more sensitive than immunohistochemistry.1 Gross lesions consist of multiple pinpoint white foci on the spleen, liver, and lymph nodes. As a facultative intracellular parasite, it may persist for years as a latent infection.11 The genes for several virulence factors have been identified and shown to share some features with the intracellular parasite, Listeria monocytogenes.1 Tularemia in other mammalian species such as horses and sheep are often associated with heavy infestation by ticks such as Dermacenter andersoni and Amblyoma americanum.12 One serologic survey indicated 12 – 24 percent of cats had antibodies to F. tularensis due to natural exposure.6 Those serologically positive animals were negative for F. tularensis DNA, indicating infection may be may have been cleared naturally. Tularemia should be considered in a differential diagnosis of unexplained febrile illness in cats.
Amyloidosis in the spleen is considered an incidental finding in this case. Amyloidosis of splenic germinal centers is reportedly a rare finding in cats and particularly domestic shorthair cats.11 Generalized amyloidosis is more common in Abyssinian cats and has been reported in Siamese cats and wild black-footed cats as well.9
Contributing Institution:
Veterinary Diagnostic Center, University of Nebraska-Lincoln, Lincoln, NE. vbms.unl.edu/nvdls
JPC Diagnosis: Spleen, lymphoid tissue: Necrosis, diffuse, with multifocal red pulp necrosis, hemorrhage, fibrin, and edema.
JPC Comment: Francisella tularensis is a global pathogen that has been classically divided into three distinct subspecies: F. tularensis subsp. tularensis, F. tularensis subsp. holarctica, and F. tularensis subsp. mediasiatica. A fourth, F. tularensis subsp. novicida, which is non-pathogenic for humans, was recently separated into a separate species, F. novocida, and is extensively used in Francisella research.7 Additional research has divided F. tularensis into genetically distinct clades, Type A and Type B, with the highly pathogenic type A (F. tularensis subsp. tularensis) found in the USA and the less virulent type B (F. tularensis subsp. holarctica) found throughout the Northern Hemisphere.7 The first report of F. tularensis subsp. holarctica in Australia was recently published.3 Both Type A and Type B infections of F. tularensis subsp. tularensis have been identified in cats.12
The first case of Francisella tularensis-mediated disease was recognized as a plague-like illness in rodents during an outbreak in Tulare County, California in 1911, and the first human cases were described three years later in two patients in Ohio, who had recently had contact with wild rabbits.4 In 1919, Dr. Edward Francis established that the disease was caused by Bacterium tularensis (named for the county in which the disease was originally identified) and appended the name “tularemia” to the disease.4 The number of cases of tularemia in the US peaked in 1939-1940 with 2,291 documented cases, but has decreased to a current average of 125 cases over the last two decades.8 Tularemia cases in both humans and animals have been identified in 49 US states; with Hawaii being the only exception.8
While the transmission of tularemia has classically been associated with contact with rodents and rabbits, giving rise to the colloquialisms “rabbit fever”, “market men’s disease”, and “meat-cutter’s disease”7, a number of important methods of transmission also exist: transmission by insect vectors including ticks, biting flies, and mosquitoes, and water-borne outbreaks.7 Aerosol-born outbreaks have accounted for some of the largest number of cases, from a 1967 outbreak and a forming area of Sweden involving more than 600 individuals7, as well as a more recent outbreak in Martha’s Vineyard, which involved 15 individuals and 1 fatality, with a common factor of lawn mowing or brush cutting a week prior to illness4.
There are three basic clinical syndromes associated with tularemia: pneumonic (exemplified by aerosol born disease as previously described), typhoidal, and ulceroglandular. Typhoidal tularemia is the result of entry of the bacteria into breaks in the skin or mucous membranes, with vascular dissemination, sepsis, and necrosis in any involved organs (as exemplified in this case). Approximately three quarters of tularemia cases in the US are localized, manifested by a tender regional lymph node a local papule which involves to a chancriform lesion; such cases are referred to as ulceroglandular tularemia.12 A single case of localized cutaneous infection resembling ulceroglandular disease has been reported in a cat with localized swellings of the right submandibular salivary gland and mandibular lymph node but lack signs of systemic illness.10
Classic association with rodents and rabbits is woefully inadequate to describe the potential for transmission from infected mammalian species. Bites, especially from asymptomatic individuals other than cats may transmit the disease, and human cases have been documented from the bites of dogs, coyotes, raccoon, skunks, opossums, squirrel monkeys, tamarins, and swine.2
- tularensis has an interesting method of surviving macrophage ingestion. Following internalization into phagocytic cells, the bacilli reside in membrane-bound endosomal compartment known as the F. tularensis-containing vacuole (FCV) which acquires both early-and late-lysosomal markers but not acid hydrolase cathepsin-D, preventing its fusion with lysosomes, and escaping the bactericidal respiratory burst.7
Within the laboratory, F. tularensis requires the use of a minimum standard biosafety level II has recommended by the American Society for microbiology. Biosafety level III facility some protocols are recommended for handling. All suspect cultures are isolated, experimental animal studies, and animal autopsies.8
References:
- Brotcke A, Weiss DS, Kim CC, Chain P, Malfatti S, Garcia E, Monack DM: Identification of MglA-regulated genes reveals novel virulence factors in Francisella tularensis. Infect Immun 74: 6642-6655, 2006
- Chomel BB, Morton JA, Kastern RQ, Chang CC. First pediatric case of tularemia after a coyote bite. Case Rep Inf Dis 2016; http://dx.doi.org/10.11.44/2016/8095138.
- Eden JS, RoseK, Ng J, Shi M, Wang Q, Sintchenko, V HolmesEC. Francisella tularensis ssp. holarctica in ringtail possums, Australia. Emerg Infect Dis 2017; 23(7):1198-1201.
- Feldman KA, enscore RE, Lathrop SL, Matyas BT, McGuill M, Schriefer ME, Stiles-Enos D, Dennis DT, Petersen LR, Hayes EB. An outbreak of primary pneumonia tularemia on Marhta's Vineyard. N Engl J Med 2001, 345(22): 1601-1606.
- Inzana TJ, Glindemann GE, Snider G, Gardner S, Crofton L, Byrne B, Harper J: Characterization of a wild-type strain of Francisella tularensis isolated from a cat. J Vet Diagn Invest 16: 374-381, 2004
- Magnarelli L, Levy S, Koski R: Detection of antibodies to Francisella tularensis in cats. Res Vet Sci 82: 22-26, 2007
- Pechous RD, McCarthy TR, Zahrt TC: Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol Mol Biol Rev 73: 684-711, 2009
- Stidham RA, Freeman DB, Tostenson SD Epidemiological review of Francisella tularensis: A case study in the complications dual diagnoses. PLoS Curr doi: 10.1371/currents.outbreaks.8eb0b55f377abc2d250314bbb8fc9d6d.
- Terio KA, O'Brien T, Lamberski N, Famula TR, Munson L: Amyloidosis in black-footed cats (Felis nigripes). Vet Pathol 45: 393-400, 2008
- Valentine BA, DeBey BM, Sonn RJ, Stauffer LR, Pielstick LG: Localized cutaneous infection with Francisella tularensis resembling ulceroglandular tularemia in a cat. J Vet Diagn Invest 16: 83-85, 2004
- Valli VEO: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, 5th ed., vol. 3, Saunders Selsevier, Philadelphia, PA, 2007
- van der Linde-Sipman JS, Niewold TA, Tooten PC, de Neijs-Backer M, Gruys E: Generalized AA-amyloidosis in Siamese and Oriental cats. Vet Immunol Immunopathol 56: 1-10, 1997
- Weinberg AN, Branda JA: Case records of the Massachusetts General Hospital. Case 31-2010. A 29-year-old woman with fever after a cat bite. N Engl J Med 363: 1560-1568, 2010