CASE I: S1335/17 (JPC 4118308)
Signalment:
4-year-old ewe, Merino sheep, sheep, Ovis aries
History:
In September 2017, this ewe was presented to a veterinary clinic showing circling and episodes of unconsciousness. Additionally, the shepherd noticed reduced water intake and appetite as well as gnashing of teeth for a few days. A referring veterinarian further diagnosed reduced pupillary reflexes and blindness. Therapy with steroids and antibiotics was ineffective.
At presentation, the animal was mildly emaciated and showed severe ataxia, tachycardia and tachypnea, reduced skin sensibility at the head, blindness and reduced reflexes. Body temperature was 39.5° C. A blood sample was analyzed and showed a mild increase of cells (predominantly monocytes/macrophages). Symptomatic therapy with dexamethasone, penicillin, vitamin B1, and tetanus serum was begun; the animal died suddenly two days later.
Gross Pathology:
Full necropsy was performed. The ewe was pregnant and in good body condition (body mass 86 kg). The carcass was in advanced decomposition. A mild ascites (100 ml), hydrothorax (80ml) and hydropericardium were noticed. Meninges were diffusely reddened with acute congestion of the vessels. There was an abscess of 1.5 cm in diameter within the left lung lobe and a marked diffuse alveolar edema. Other organs and tissue were unaltered beside marked decomposition.
Laboratory results:
White blood cells: 13.2 G/l (ref. 4.2-6.2 G/l) = mild leukocytosis
Urea: 10.2 mmol/l (ref. 5 mmol/l) = mild azotemia
Transketolase activity: 8% (ref. <50%)
Blue tongue virus (PCR): negative
Pestivirus (PCR): negative
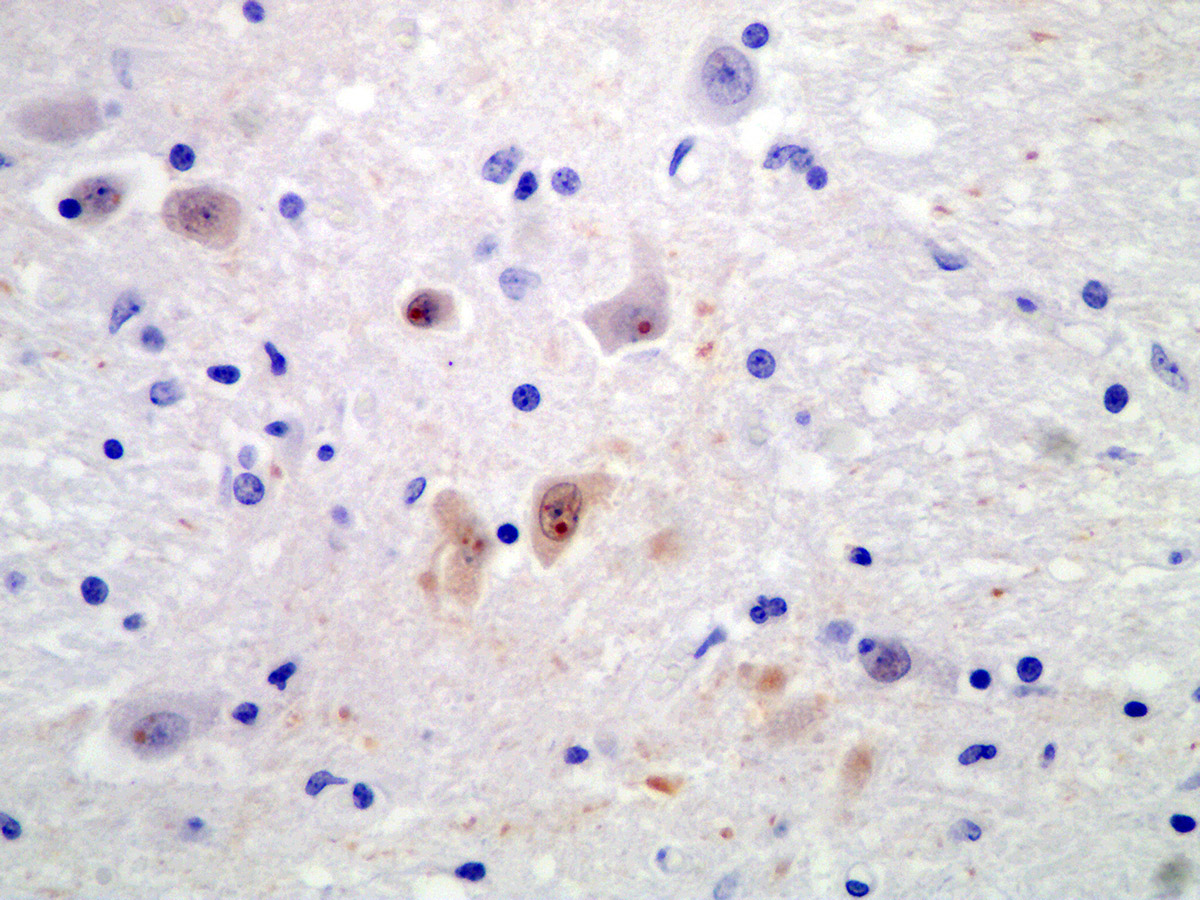
Immunohistochemically bornavirus antigen (BO18) was detected within the cytoplasm of neurons and astrocytes with well discernable immunopositive Joest-Degen inclusion bodies.
Microscopic Description:
Brain (cerebrum and brain stem):
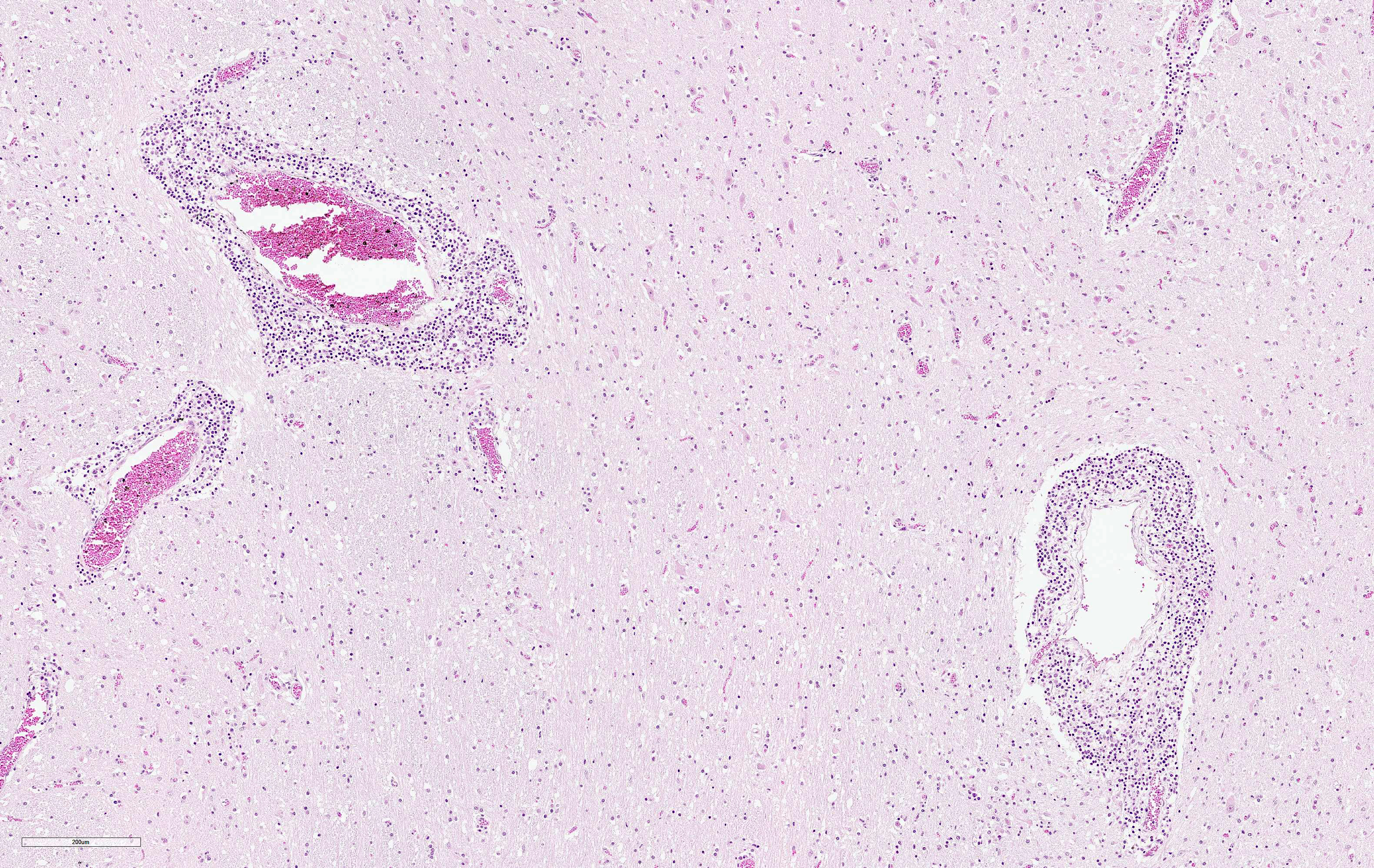
Affecting 60% of Virchow-Robin spaces are expanded by perivascular cuffs composed of 1 to 8 layers of lymphocytes, plasma cells and fewer macrophages. Meninges are similarly affected. Multifocally, neurons are shrunken, hypereosinophilic (neuronal necrosis), and surrounded by increased numbers of glial cells (satellitosis). The number of glial cells is increased throughout tissue section sometimes forming nodular aggregates (gliosis). Rarely, neurons contain poorly discernably 4-6 μm, round, eosinophilc Cowdry-type-B inclusion bodies (Joest-Degen bodies)
Contributor's Morphologic Diagnoses:
Brain, meningoencephalitis, lymphoplasmacytic, diffuse, marked
with perivascular lymphohistiocytic cuffs, multifocal neuronal degeneration and
necrosis, gliosis, and neuronal eosinophilic intranuclear viral inclusion
bodies, etiology consistent with Borna disease, ovine.
Contributor's Comment:
This ovine case is a classic example of Borna disease, first described in horses in 1813 and named after the town Borna in Saxony/Germany, due to a local severe outbreak in military horses in 1894.3 The etiologic agent is borna disease virus-1 (BoDV-1), a single-stranded negative-orientated RNA-virus, order Mononegavirales. After the discovery of several new members of the virus family Bornaviridae in recent years, the International Committee on Taxonomy of Viruses has proposed a new taxonomy in 2018.1 Now the family Bornaviridae consists of two genera: the genus Carbovirus with members infecting snakes (carpet pythons in Australia) and the genus Orthobornavirus, including mammalian-1 orthobornavirus with BoDV-1 as the classic prototype, and several other virus species. Mammalian-2 orthobornavirus includes the variegated squirrel Bornavirus-1 (VSBV-1) with a reservoir in certain squirrels (Sciurus variegatoides, Callosciurus sp.)3 Infections with VSBV-1 have been linked to fatal cases of human encephalitis in squirrel breeders.13 Psittaciform-1 orthobornavirus is the etiologic agent of psittacine proventricular dilatation disease.8 Additionally, several other viral species have been found in different wild birds.1
Classical Borna disease mainly occurs in horses and sheep, but can occasionally affect other warm-blooded animals.14 The first outbreaks at the end of the 19th century were epidemically, but in recent years, only sporadic cases in endemic regions occur. Clinical signs of Borna disease include therapy-resistant fever, hyperesthesia, pharyngeal paralysis, circling, muscular tremors, spasm and blindness, drowsiness and flaccid paresis. Incubation time is about 2-6 months, after onset of clinical signs animal usually die within 4 weeks. Mortality reaches 90%-100%. 3, 14
BoDV-1 is neurotropic, likely uses a nasal infection route and enters the brain via transaxonal migration along the olfactory bulb. Within the CNS, the virus shows widespread distribution of viral antigens in neurons, astrocytes, ependymal cells and oligodendrocytes. In later stages, highest viral loads can be detected in the grey matter of the limbic system, the hippocampus, in basal ganglia and brainstem. BoDV-1 replicates in the nucleus of the host cells and does not cause any direct cytopathic effect. Brain lesions are induced by immune-mediated mechanisms. Affected animals do not develop obvious gross lesions, but develop a fulminant non-suppurative polioencephalitis with occasional involvement of meninges. Lesions include thick perivascular cuffs composed of lymphocytes, plasma cells and macrophages, neuronal necrosis, satellitosis and gliosis. Rarely, Cowdry type B intranuclear eosinophilic inclusion bodies (Joest-Degen inclusion bodies) can be found.3 In this case, they were easily visible immunohistochemically but not by using Giemsa stain.
The epidemiology of Borna disease, with occurrence in endemic areas in Central Europe (Germany, Switzerland, Austria, Liechtenstein), higher incidence in certain years and seasons (spring and summer) and higher incidence on farms with low hygiene and rodent control, points towards a rodent reservoir.5 Until now, the only confirmed reservoir of BoDV-1 is the bicolored white-toothed shrew (Crocidura leucodon).2, 6, 7 In shrews, BoDV-1 can be found in the nervous system and additionally in many peripheral organs including excretory organs (skin glands, kidney). In this reservoir species, there is no inflammation associated with the virus and infected shrews do not show clinical signs. 11
The zoonotic potential of BoDV-1 has been controversial for the last thirty years. An association with neuropsychiatric diseases has been repeatedly suggested.4 However, in 2018, few cases of encephalitis caused by BoDV-1 infection in human patients receiving donated organs support the hypothesis of a zoonotic potential of BoDV-1.15
Contributing Institution:
Institut fuer Veterinaer-Pathologie, Justus-Liebig-Universitaet Giessen
Frankfurter Str. 96, 35392 Giessen, Germany
http://www.uni-giessen.de/cms/fbz/fb10/institute_klinikum/institute/pathologie
JPC Diagnosis:
Cerebrum: Meningoencephalitis, lympho-histiocytic, diffuse, moderate, with rare neuronal necrosis and intranuclear viral inclusions.
JPC Comment:
The contributor provides a concise review of recent Bornaviridae taxonomy updates as well as details of its pathogenesis in its reservoir host, the bicolored white-toothed shrew (Crocidura leucodon), and higher mammals dead-end hosts which demonstrate meningoencephalitis. 10
As noted by the contributor, classical Borna disease is typically associated with horses and sheep, although other warm blooded animals may also be infected. Interestingly, New World camelids have recently been identified as potential sentinels for BoDV-1, with reports of symptomatic cases in these species while other species are lacking in similar geographic regions over the same time periods.10
An example of camelid infection was demonstrated on a Swiss farm that maintained a herd of up to 8 breeding llamas in addition to cattle, horses, goats, cats, dogs, rabbits, and chickens. Over a two-decade period, 12 of 30 breeding llamas succumbed to Borna disease, but no other species kept on the farm were affected. Abnormal prehension of food was the first reported clinical sign, followed by separation from the herd, shade-seeking, head rubbing against the ground, and biting inanimate structures. Animals in late stages of the disease ran into fences or walls with signs of visual impairment. In addition, the animals tended to constantly move but did not exhibit aimless circling as seen in horses.10
A similar phenomenon occurred in southern Germany, near the Swiss border. Between 2002 and 2008, there were five reported cases of Borna disease, three of which occurred in alpaca herds (the other two cases were a sheep and horse). Surprisingly, this region had been dormant for Borna disease for several decades prior to these cases. In 2011, BoDV-1 was identified via PCR in a bicolored white-toothed shrew in the same region. Complete genome sequencing found the BoDV-1 in the shrew was between 96.6-98.2% identical to those obtained from infected animals.10
As previously mentioned by the contributor, BoDV-1 cases are typically seasonal, with most occurring in the in spring and summer months. This is most likely attributed to the biologic behavior of the infected bicolored white-toothed shrews (Crocidura leucodon) which are more active during these times. Notably, historical temperature data for the region of the previously discussed llama farm in Switzerland demonstrated increasing temperatures over the last decade with 2011, 2012, 2014, 2015, 2017, 2018, and 2019 being particularly warm. Borna disease outbreaks occurred in six of these seven "hot" years but not in the cooler years of 2013 and 2016. Therefore climate change may present a significant contributing factor in regard to incidence of this disease.10
Joest-Degen bodies, also known as Cowdry type B bodies, are named after their discoverer, who found the intranuclear inclusions to be pathognomonic for Borna disease in the horse in 1909.9,12
There was significant slide variability associated with this submission. Conference participants noted sections containing hippocampus contained abundant Joest-Degen bodies, which were rarely observed in other sections.
References:
1. Amarasinghe GK, Arechiga Ceballos NG, Banyard AC, et al. Taxonomy of the order Mononegavirales: update 2018. Arch Virol. 2018; doi: 10.1007/s00705-018-3814-x
2. Bourg M, Herzog S, Encarnacao JA, Nobach D, Lange-Herbst H, Eickmann M, Herden C. Bicolored white-toothed shrews as reservoir for borna disease virus, Bavaria, Germany. Emerg Infect Dis. 2013;19:2064-2066.
3. Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 6th ed. Vol 1. Elsevier, St. Louis, Missouri; 2016:250-406.
4. Dürrwald R, Kolodziejek J, Herzog S, Nowotny N. Meta-analysis of putative human bornavirus sequences fails to provide evidence implicating Borna disease virus in mental illness. Rev Med Virol. 2007;17:181-203.
5. Dürrwald R, Kolodziejek J, Muluneh A, Herzog S, Nowotny N: Epidemiological pattern of classical Borna disease and regional genetic clustering of Borna disease viruses point towards the existence of to-date unknown endemic reservoir host populations. Microb Infect. 2006;8:917-929.
6. Dürrwald R, Kolodziejek J, Weissenbock H, Nowotny N. The bicolored white-toothed shrew Crocidura leucodon (HERMANN 1780) is an indigenous host of mammalian Borna disease virus. PLoS One 2014;9:e93659.
7. Hilbe M, Herrsche R, Kolodziejek J, Nowotny N, Zlinszky K, Ehrensperger F. Shrews as reservoir hosts of borna disease virus. Emerg Infect Dis. 2006;12:675-677.
8. Hoppes SM, Tizard I, Shivaprasad HL. Avian bornavirus and proventricular dilatation disease: diagnostics, pathology, prevalence, and control. Vet Clin North Am Exot Anim Pract. 2013;16:339-355.
9. Joest E, Degen K. Über eigentümliche Kerneinschlüsse der Ganglienzellen bei der enzootischen Gehirn-Rückenmarksentzündung der Pferde. Z Inf Krkh Haustiere. 1909;6:348?356.
10. Malbon AJ, Dürrwald R, Kolodziejek J, et al. New World camelids are sentinels for the presence of Borna disease virus [published online ahead of print, 2021 Jan 27]. Transbound Emerg Dis. 2021;10.1111/tbed.14003.
11. Nobach D, Bourg M, Herzog S, Lange-Herbst H, Encarnacao JA, Eickmann M, Herden C. Shedding of Infectious Borna Disease Virus-1 in Living Bicolored White-Toothed Shrews. PLoS One. 2015;10:e0137018.
12. Sasaki S, Ludwig H. In borna disease virus infected rabbit neurons 100 nm particle structures accumulate at areas of Joest-Degen inclusion bodies. Zentralbl Veterinarmed B. 1993;40(4):291-297.
13. Schlottau K, Hoffmann B, Homeier-Bachmann T, Fast C, Ulrich RG, Beer M, Hoffmann D. Multiple detection of zoonotic variegated squirrel bornavirus 1 RNA in different squirrel species suggests a possible unknown origin for the virus. Arch Virol. 2017;162:2747-2754.
14. Vandevelde M, Higgins RJ, Oevermann A. Veterinary Neuropathology. Wiley-Blackwell, Oxford, UK; 2012:57-58.
15. Epidemiol. Bulletin 10/2018, Robert Koch Institute Berlin, Germany, https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2018/Ausgaben/10_18.pdf?__blob=publicationFile).