CASE I: RP 18046 (JPC 4153154)
Signalment: Juvenile, male Western Turkey Vulture (Cathartes aura aura)
History:
The vulture was found down and unable to rise or fly. Physical exam showed severe paresis and absence of pain sensation in both legs. Due to poor prognosis for return to the wild, humane euthanasia was elected.
Gross Pathology:
Numerous variably sized, well demarcated, white to tan nodules were randomly scattered throughout the kidneys and intestine.
Laboratory Results:
PCR (frozen kidney):
- Avian Poxvirus REV LTR flanking region: positive with 100% identity to Vultur gryphus poxvirus genome (GenBank: AY246559)
- Avian Poxvirus core P4b protein gene: positive with 100% identity to Avipoxvirus isolate Hawaii isolate P62 4b core protein gene (GenBank: KC0180210)
Microscopic Description:
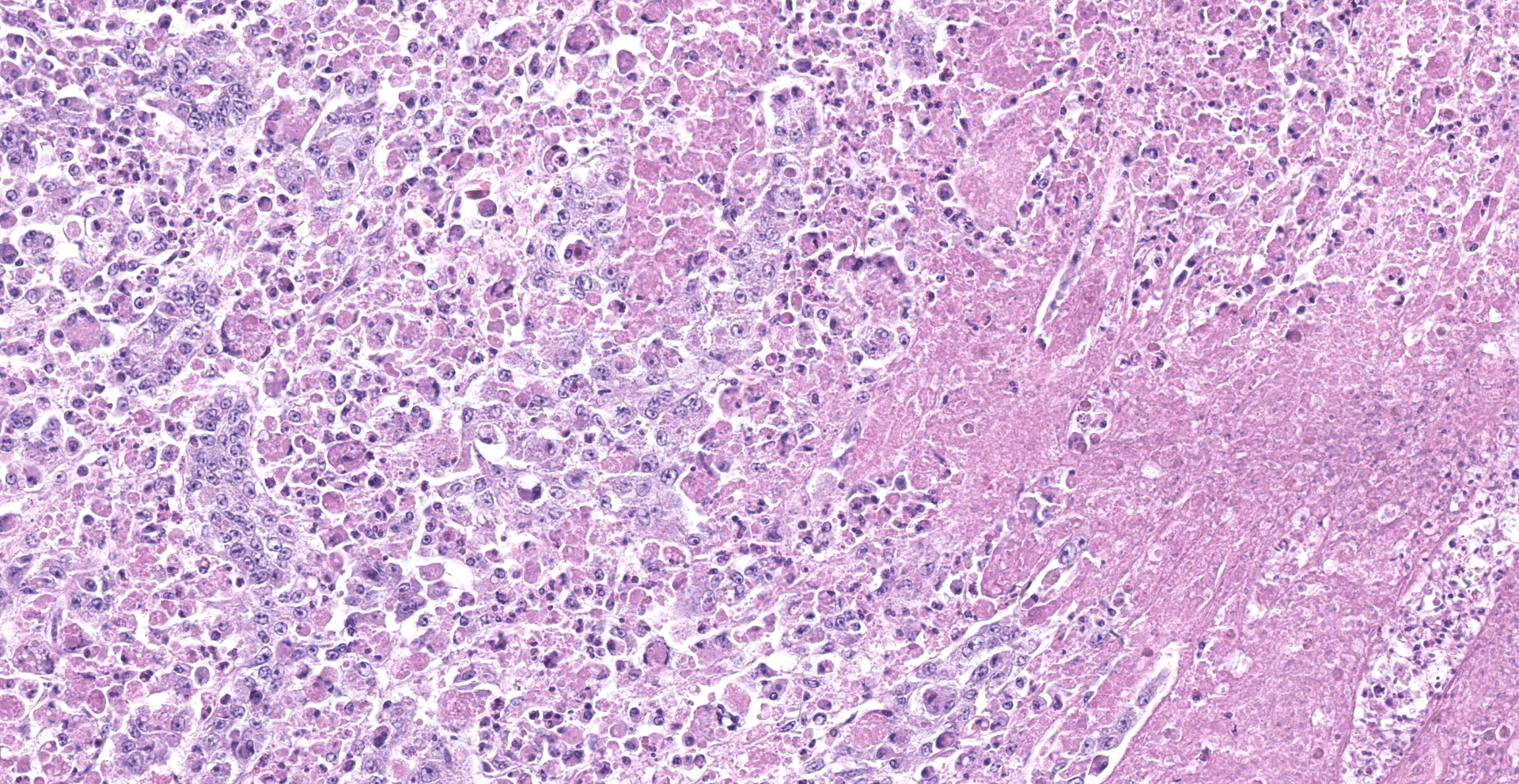
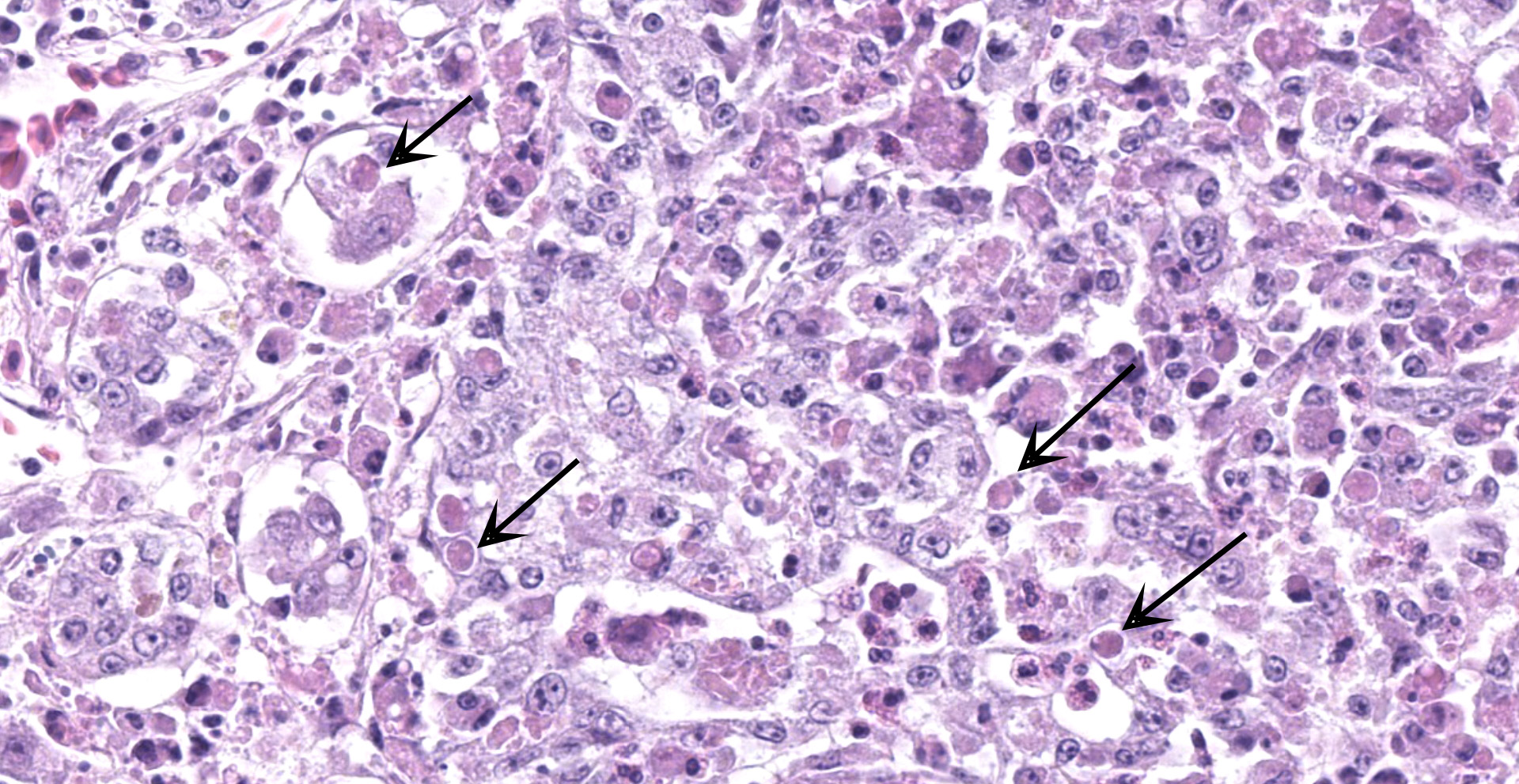
Kidney: One section of kidney is examined. Approximately 80% of the renal parenchyma is effaced by a dense inflammatory infiltrate that extends throughout the interstitium, surrounding and replacing tubules and glomeruli. The inflammation is composed predominantly of macrophages with fewer lymphocytes, plasma cells, heterophils, and multinucleated giant cells. Macrophages frequently contain brightly eosinophilic, cytoplasmic material that varies in appearance from unstructured, granular to globular material to discrete, round inclusions with a central clear zone reminiscent of Bollinger bodies. Multifocal areas of necrosis are present throughout the inflammatory infiltrate, characterized by loss of cellular detail and architecture with hypereosinophilic and karyorrhectic cellular debris.
Not included on this slide:
Similar inflammation with intrahistiocytic, intracytoplasmic inclusions were present in the spleen, adrenal glands, thyroid glands, proventriculus, intestine, pancreas, cloaca, and bone marrow. Areas of epithelial hyperplasia with characteristic Bollinger bodies were present in the esophageal mucosa and skin surrounding the vent.
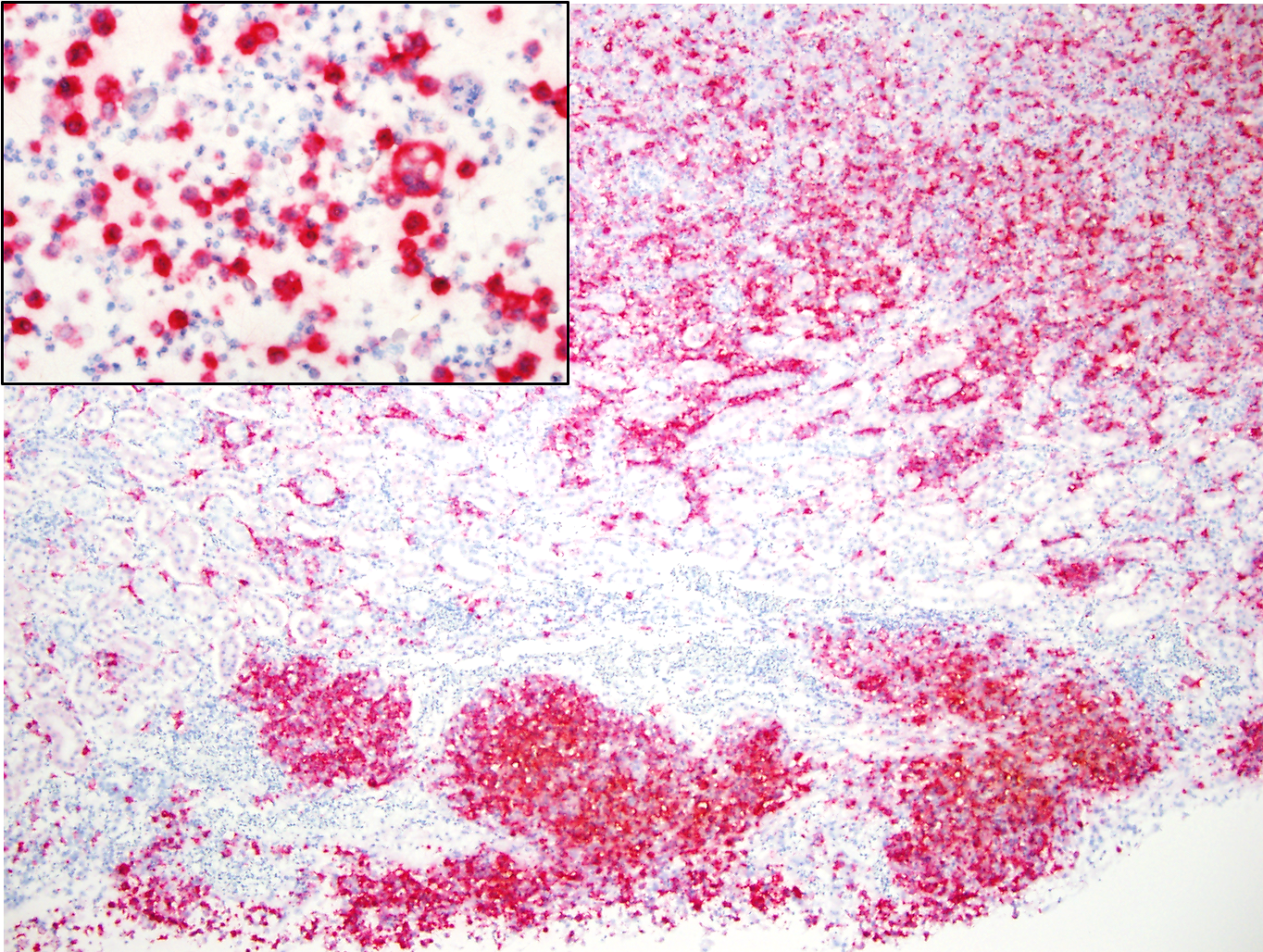
In-situ Hybridization for Avian Poxvirus core P4b protein gene (kidney): diffuse positive labeling within macrophages.
Contributor's Morphologic Diagnoses:
Kidney: marked, subacute, diffuse, granulomatous and necrotizing nephritis with intrahistiocytic viral inclusions (avian poxvirus by PCR and in-situ hybridization)
Contributor's Comment:
Death of this juvenile turkey vulture was due to widespread granulomatous inflammation affecting multiple organs. Macrophages within this inflammation often contained intracytoplasmic inclusions that often resembled typical poxvirus-associated Bollinger bodies. In addition to this inflammatory process within the visceral organs, typical proliferative poxviral lesions were present in the esophagus and skin surrounding the vent. Two PCR assays targeting different regions of the avian poxvirus genome (REV LTR flanking region and core P4b protein gene) detected the presence of avian poxvirus DNA in frozen kidney from this turkey vulture. An in-situ hybridization probe designed to target the avian poxvirus core P4b protein gene also detected the presence of avian poxvirus DNA within infiltrating macrophages in the kidney. Based on the histopathologic and molecular findings, the cause of the widespread inflammatory lesions was attributed to a systemic infection with avian poxvirus. The limb paresis noted clinically in this turkey vulture was due to extension of the inflammation from the kidneys along the sciatic nerves.
Avian poxviruses are DNA viruses in the family Poxviridae, subfamily Chordopoxviridae, genus Avipoxvirus that affect a wide range of bird species. In commercial poultry productions, avian pox is of economic importance due to decreased egg production, stunted growth, and variable mortality rates in infected flocks.14 Avian poxvirus infections are classically divided into two forms: cutaneous or "dry" pox, and diphtheritic or "wet" pox. Lesions associated with dry pox include one to multiple proliferative, crusty to ulcerated nodules on the non-feathered skin. Birds with wet pox develop proliferative plaques or caseous pseudomembranes on the mucosa of the oropharynx, esophagus, and upper respiratory tract.14 Both forms of avian poxvirus share similar histologic findings including epithelial hyperplasia and ballooning of epithelial cells with large, cytoplasmic, eosinophilic viral inclusions referred to as Bollinger bodies.14
A third form, systemic avian pox, has been rarely reported and most commonly causes a widespread respiratory infection in canaries with high mortality rates.14 Affected canaries develop fibrinous pneumonia, tracheitis, and air sacculitis with proliferative respiratory epithelial cells containing eosinophilic, intracytoplasmic inclusions.1,2,8,11,12 Additionally, inclusions were also identified in splenic and thymic reticuloendothelial cells1, and mononuclear cells in the thymus, bursa of Fabricius, spleen, and bone marrow.12 Similar avian pox-associated respiratory infections have also been reported in sparrows2,4 and rosy-faced lovebirds15, in which inclusions were also identified in coelomic serosal cells4 and in the bone marrow, bursa of Fabricius, and mononuclear cells within the bones of the digits and skull.15
Systemic avian pox can cause predominantly non-respiratory infections as well. Kim et al. 2003 described a captive Andean condor with granulomatous nodules in the heart, lung, liver, kidney, small intestine, pancreas, and spleen. Inclusions were identified within macrophages in these nodules as well as in biliary epithelium, splenic reticuloendothelial cells, and thymic and bursal epithelial cells. Similar nodular granulomatous inflammation within parenchymal organs with intrahistiocytic inclusions has been seen in other wild and captive Cathartiformes, including the present case, as well as in multiple species of Passerines, Coraciiformes, and Cuculiformes (Sinnott et al., submitted for publication).
Ten species of avian poxvirus are currently recognized by the International Committee on Taxonomy of Viruses: fowlpox virus, canarypox virus, juncopox virus, mynahpox virus, pigeonpox virus, psittacinepox virus, quailpox virus, sparrowpox virus, starlingpox virus, and turkeypox virus (www.ictvonline.org), although numerous strains exist. Phylogenetic studies using sequences of the core P4b protein gene divides avian poxviruses into three clades: clade A (fowlpox viruses), clade B (canarypox viruses), and clade C (psittacinepox viruses).6 It is speculated that systemic avian poxvirus disease is associated with infection by strains in the B1 subclade (Sinnott et al., submitted for publication).1
The exact pathogenesis of systemic pox in birds is not fully understood, and additional work on host factors, epidemiology of infection, and genomic analysis is ongoing.
Systemic involvement of poxviruses is rare in domestic animals with the exception of sheeppox and goatpox, which cause systemic infections often with high mortality in susceptible flocks.7 Transmission of these viruses occurs via inhalation or skin abrasions, followed by systemic viremia. Affected sheep and goats develop typical poxviral cutaneous lesions in sparsely fleeced areas as well as dermal and subcutaneous edema, vasculitis, proliferative alveolitis and bronchiolitis, and gastrointestinal ulceration. Visceral organs such as the heart, kidney, liver, adrenal gland, thyroid gland, and pancreas are infiltrated by sheeppox cells, mononuclear cells characterized by intracytoplasmic viral inclusions and nuclei with vacuolated chromatin.7
Contributing Institution:
San Diego Zoo Global
Disease Investigations
P.O. Box 120551
San Diego, CA 92112-0551
https://institute.sandiegozoo.org/disease-investigations
JPC Diagnosis:
Kidney: Nephritis, tubulointerstitial, necrotizing and granulomatous, diffuse, severe, with marked tubular loss and numerous intracytoplasmic viral inclusions (Bollinger bodies).
JPC Comment:
The contributor provides an outstanding review of avipoxviruses and their characteristic manifestations of cutaneous, diphtheritic, and systemic disease in avian species.
Route of transmission plays a significant role toward the development of the diphtheritic and cutaneous forms of the disease. The cutaneous or "dry" form of avian pox is commonly transmitted by arthropod vectors but can also be directly transmitted between infected and susceptible birds. Indirect transmission may also occur through contaminated water and food as well as contact with fomites. In contrast, the diphtheritic or "wet" form of avian pox occurs following inhalation of the virus. Although aerosol transmission occurs less commonly than via direct contact, birds housed in close confinement situations such as aviaries or rehabilitation facilities are at increased risk of developing diphtheritic avian pox.5
Interestingly, poxviruses indirectly played a major role in the establishment of the United States Department of Agriculture as the regulatory agency for veterinary biologic products. The US Congress passed the Virus-Serum-Toxin (VST) Act in 1914 following an outbreak of foot-and-mouth disease that had been traced to contaminated smallpox vaccine virus that had been imported from Japan. The VST Act authorized the Secretary of Agriculture to prevent the preparation and marketing of worthless, contaminated, dangerous, or harmful virus, serum, toxin, or analogous products. The first license issued for an avian product following passage of the VST Act was provided to the University of California, Berkley on January 13th, 1916 for a fowlpox vaccine labeled "for the prevention of chicken pox."3
Mosquitoes, especially Culex and Aedes spp. are commonly implicated as mechanical vectors of avipoxviruses and are able to retain viable virus on the proboscis for at least 14 days after feeding on an infected bird. The mosquito then mechanically transmits the virus to additional susceptible birds during subsequent blood meals, allowing it to serve as a key bridge between reservoirs and na?ve hosts. In addition to mosquitoes, biting midges and mites also been implicated as mechanical vectors of avipoxviruses.16
Poxviruses have large complex genomes encoding several genes that interfere with host-cell apoptosis mechanisms. Examples include viral Bcl-2 (vBcl-2) mimics that modulate with the intrinsic apoptosis pathway as well as other inhibitory strategies targeting the extrinsic pathway, such as TNF receptor homologs. In situations where the intrinsic apoptosis pathway is uninhibited, some viral infections initiate programmed cell death through the activation of BH3 proteins. These sensor proteins promote apoptosis through the neutralization of pro-survival Bcl-2 proteins or by directly facilitating oligmerization of pro-apoptotic proteins (e.g. Bak and Bax) on the mitochondrial outer membrane. Two vBcl-2 mimics specifically sequenced in avipoxviruses include FPV039 from fowl poxvirus (FPV) and CNP058 from canary poxvirus (CNPV). FPV039 suppresses the intrinsic apoptosis cascade through interactions with all pro-apoptotic Bcl-2 proteins, including Bax, resulting in inhibition of mitochondrial pore formation. In contrast, CNP058's interactions are restricted to a specific set of BH3 only proteins (e.g. Bim), resulting in their sequestration and preventing interaction with Bak and Bax, preventing apoptosis.13
Participants engaged in spirited discussion in regard to the presence of intracytoplasmic inclusions (i.e. Bollinger bodies) within macrophages. Although participants suspected many cells with intracytoplasmic inclusions to be macrophages, they could not definitively differentiate between macrophages and sloughed epithelial cells. Participants unanimously agreed cytoplasmic inclusions are present within epithelial cells.
In addition, the moderator utilized the conference as an opportunity to educate participants on Otto von Bollinger (1843-1090), the German pathologist credited with first describing the fowlpox inclusion bodies now bearing his name. Bollinger is also credited with describing the etiologic agent of bovine actinomycosis (Actinomyces bovis - "lumpy jaw") in 1877, providing an early description of delayed traumatic intracerebral hematoma in 1891, studies on rabies and hydrophobia, and he was a co-founder and editor of a German journal for veterinary medicine and comparative pathology.
References:
1. Avila-Reyes VA, Diaz-Morales V, Chavez-Maya F, Garcia-Espinosa G, Sanchez-Godoy FD. Outbreak of systemic avian pox in canaries (Serinus canaria domestica) associated with the B1 subgroup of avian pox viruses. Avian Dis. 2019;63:525-530.
2. Donnelly TM, Crane LA. An epornitic of avian pox in a research aviary. Avian Dis. 1984;28:517-525.
3. Espeseth DA, Lasher H. Early history of regulatory requirements for poultry biologics in the United States. Avian Dis. 2010;54(4):1146-1143.
4. Giddens WE, Swango LJ, Henderson JD, et al. Canary pox in sparrows and canaries (Fringillidae) and in weavers (Ploceidae). Vet Pathol. 1971;8:260-280.
5. Godoy LA, Dalbeck LS, Tell LA, et al. Characterization of avian poxvirus in Anna's Hummingbird (Calypte anna) in California, USA. J Wildl Dis. 2014;49(4):978-985.
6. Gyuranecz M, Foster JT, Dan A, et al. Worldwide phylogenetic relationship of avian poxviruses. J Virol. 2014;87:4938-4951.
7. Hargis AM, Myers S. The Integument. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier; 2017:1123-1124.
8. Johnson BJ, Castro AE. Canary pox causing high mortality in an aviary. J Am Vet Med Assoc. 1986;189:1445-1447.
9. Kim TJ, Schnitzlein WM, McAloose D, Pessier AP, Tripathy DN. Characterization of an avianpox virus isolated from and Andean condor (Vultur gryphus). Vet Microbiol. 2003;96:237-246.
10. Pesaro S, Biancani B, Fabbrizi G, Rossi G. Squamous cell carcinoma with presence of poxvirus-like inclusions in the foot of a pink-backed pelican (Pelecanus rufescens). Avian Pathol. 2009;38(3):229-231.
11. Reza K, Nasrin A, Mahmoud S. Clinical and pathologic findings of concurrent pox lesions and aspergillosis infection in canaries. Asian Pac J Trop Biomed. 2014;3:182-185.
12. Shivaprasad HL, Kim T, Tripathy D, Woolcock PR, Uzal F. Unusual pathology of canary poxvirus infection associated with high mortality in young and adult breeder canaries (Serinus canaria). Avian Pathol. 2009;38:311-316.
13. Suraweera CD, Hinds MG, Kvansakul M. Poxviral Strategies to Overcome Host Cell Apoptosis. Pathogens. 2020;10(1):6.
14. Tripathy DN, Reed WM. Pox. In: Swayne DE, ed. Diseases of Poultry. 14th ed. Ames, IA: Wiley-Blackwell; 2014: 333-349.
15. Tsai SS, Chang TC, Yang SF, et al. Unusual lesions associated with avian poxvirus infection in rosy-faced lovebirds (Agapornis roseicollis). Avian Pathol. 1997;26:75-82
16. Yeo G, Wang Y, Chong SM, et al. Characterization of Fowlpox virus in chickens and bird-biting mosquitoes: a molecular approach to investigating Avipoxvirus transmission. J Gen Virol. 2019;100(5):838-850.