CASE IV: N17-459 (JPC 4142728-00)
Signalment:
4-year-old, spayed-female, mixed breed dog (Canis familiaris).
History:
This dog presented for a history of perineal lesions. The tail was amputated by the referring veterinarian, after which Lagenidium sp. infection was confirmed via PCR. MRI revealed sublumbar lymphadenopathy and regional soft tissue involvement. The patient became fecally incontinent. On presentation to the University of Florida, there was thickening of the rectal wall and pain on rectal palpation. There was an initial dramatic reduction of the lesion size with therapy. However, lesions returned and worsened, with necrosis of perineal tissue. Euthanasia was elected.
Gross Pathology:
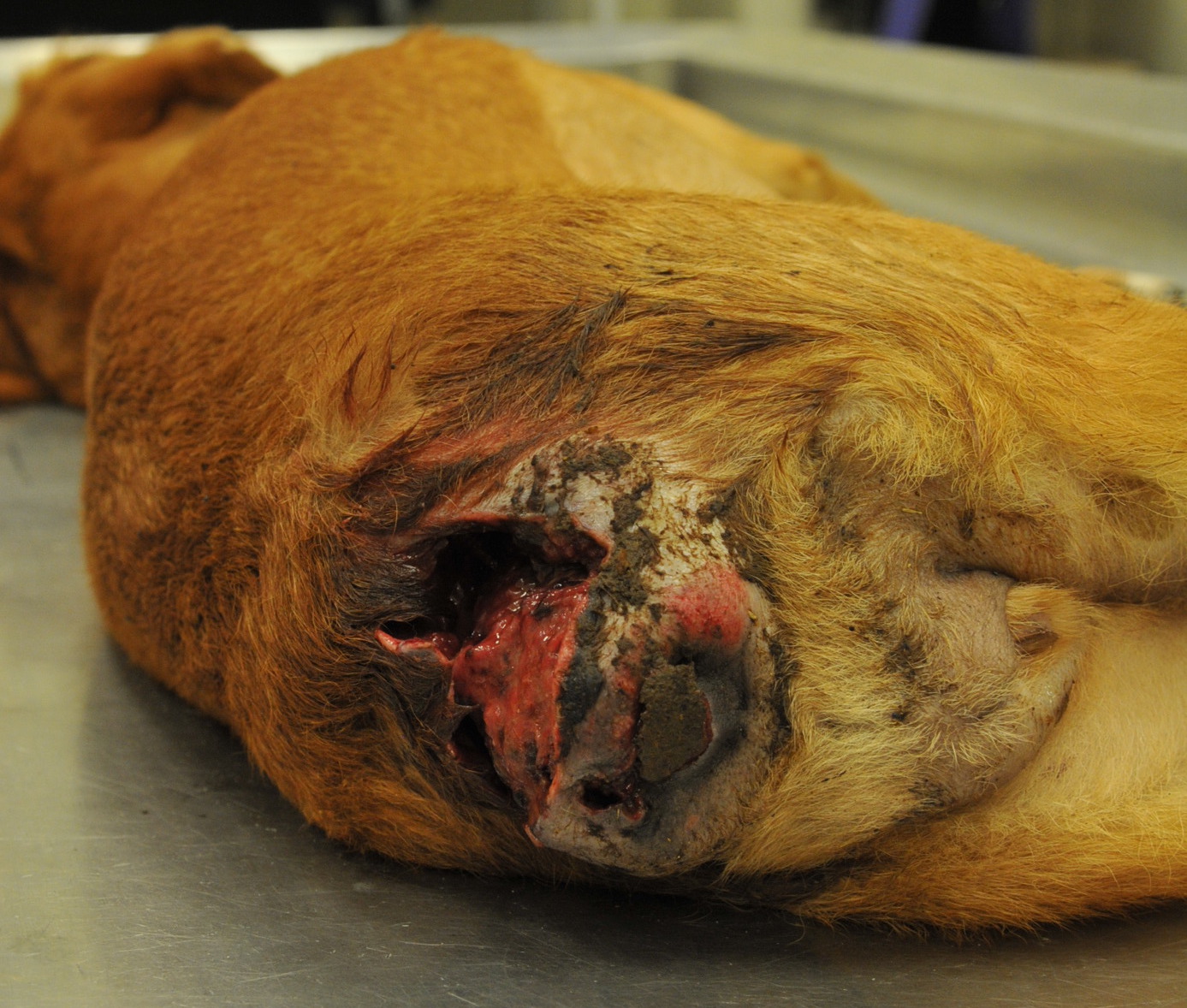
There is moderate, diffuse expansion of the subcutis of the left hind limb with yellow, gelatinous material (edema), with the proximal limb to hock region twice the circumference of the contralateral limb. There is a 12x5x3 cm nodule within the left inguinal region that is centrally cavitated and contains brown-green, watery to gelatinous material. The surrounding tissue is brown to purple, firm and irregular, and approximately 3 mm to 1 cm thick. Dorsal to the rectum there is a broadly ulcerated to deeply cavitated, 6.5x5.5 cm defect lined by pale tan to tan, soft to firm, irregular tissue that extends deep into the underlying subcutaneous tissue and connects with the dorsal pelvic canal. Adjacent to the rectum and attached to the rectal wall there are three pale tan, soft to firm, nodular to confluent masses measuring up to 7x3x3 cm within the pelvic canal as described above. On cut section these nodules are solid and mottled pale tan to tan with a granular appearance.
Laboratory results:
PCR via the RDVM detected Laginidium sp. DNA.
Microscopic Description:
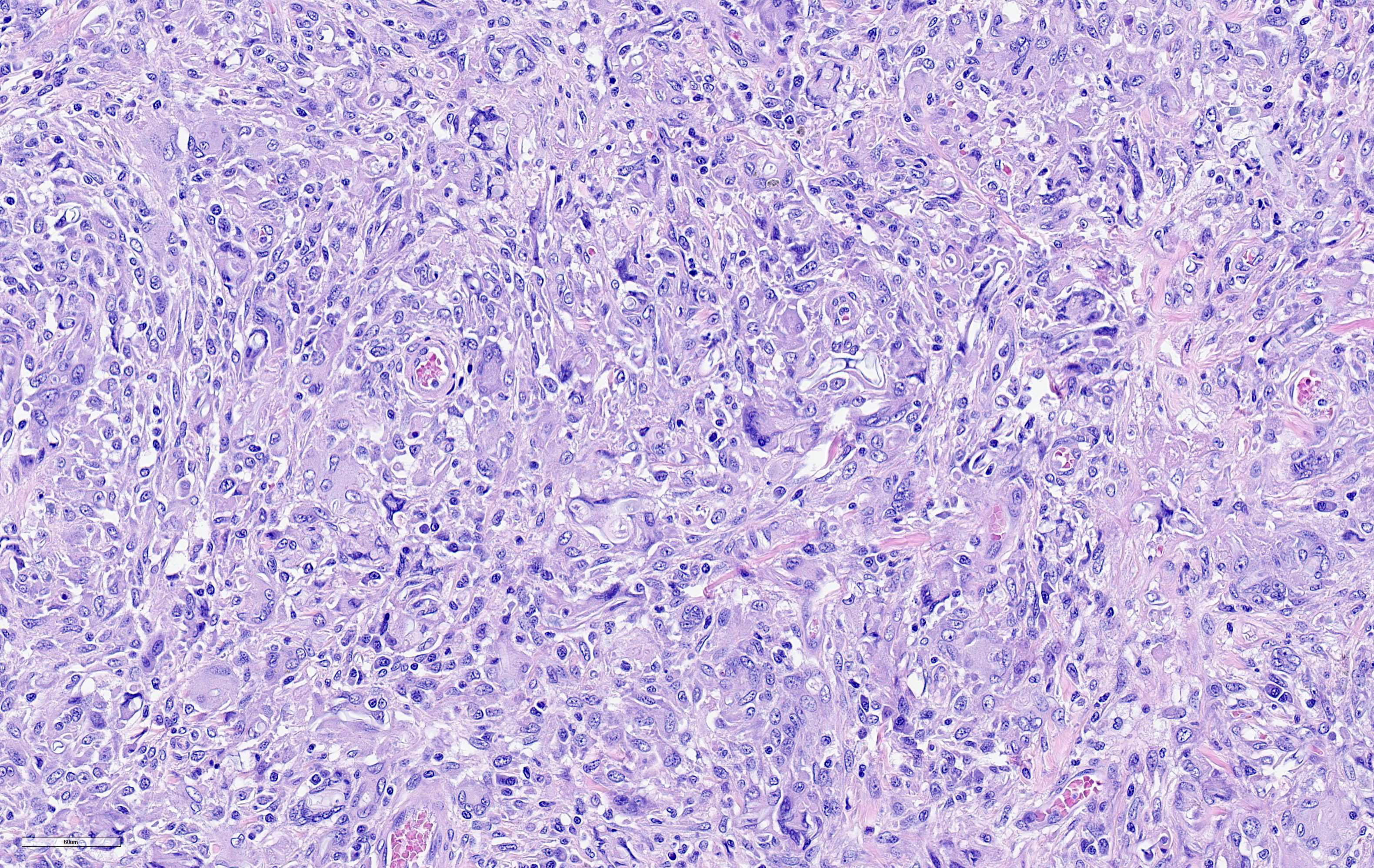
Within the dermis and subcutis of the perineum, there is a multinodular to confluent inflammatory infiltrate with a large tract lined by inflamed granulation tissue. The epidermis adjacent to the tract is ulcerated. A similar inflammatory infiltrate is present in the perianal dermis and subcutis and anal skeletal muscle, mesorectum/mesocolon and rectal muscularis, with minimal extension into the anorectal submucosa. A similar inflammatory infiltrate effaces about half of a small lymph node in the mesorectum/mesocolon. A tract is also present in the perianal tissue, with much of the tract lined by fibrin with enmeshed cellular debris and neutrophils. In one area of the tract, the defect is lined by stratified squamous epithelium. The inflammatory infiltrate is composed primarily of macrophages and multinucleated giant cells with fewer lymphocytes, plasma cells and sometimes few to low numbers of neutrophils and/or eosinophils. Within the inflammatory infiltrate, and often within the cytoplasm of multinucleated giant cells, there are large numbers of approximately 4-12 µm diameter septate, branching hyphae with non-parallel walls. Vessels within the inflammatory infiltrate sometimes to often exhibit fibrinoid necrosis, contain cell debris and/or inflammatory cells within the vessel walls, recanalization, and/or contain fibrin thrombi. There are multifocal variably sized areas of necrosis within the inflammatory infiltrate, often adjacent to vessels with vasculitis or fibrinoid necrosis. There are occasional small areas of necrosis central within nodular granulomatous infiltrates.
Contributor 's Morphologic Diagnoses:
Cellulitis, perianal/perineal
dermatitis, anoproctitis, and lymphadenitis, granulomatous to pyogranulomatous
to eosinophilic and granulomatous, chronic, locally extensive, severe, with
intralesional hyphae
Contributor 's Comment:
Lagenidium is one of a number of pathogenic oomycetes, with several species infecting mammals, insects, and nematodes, with the prevalence of Lagenidium infections in mammals increasing over the last several years.8 Lagenidium giganticum was also approved for several years as a biological control agent for mosquitoes; subsequent studies have found significant molecular similarities between mammalian Lagenidium strains and those approved for mosquito control9, although biocontrol strains did not grow well at 37˚C.
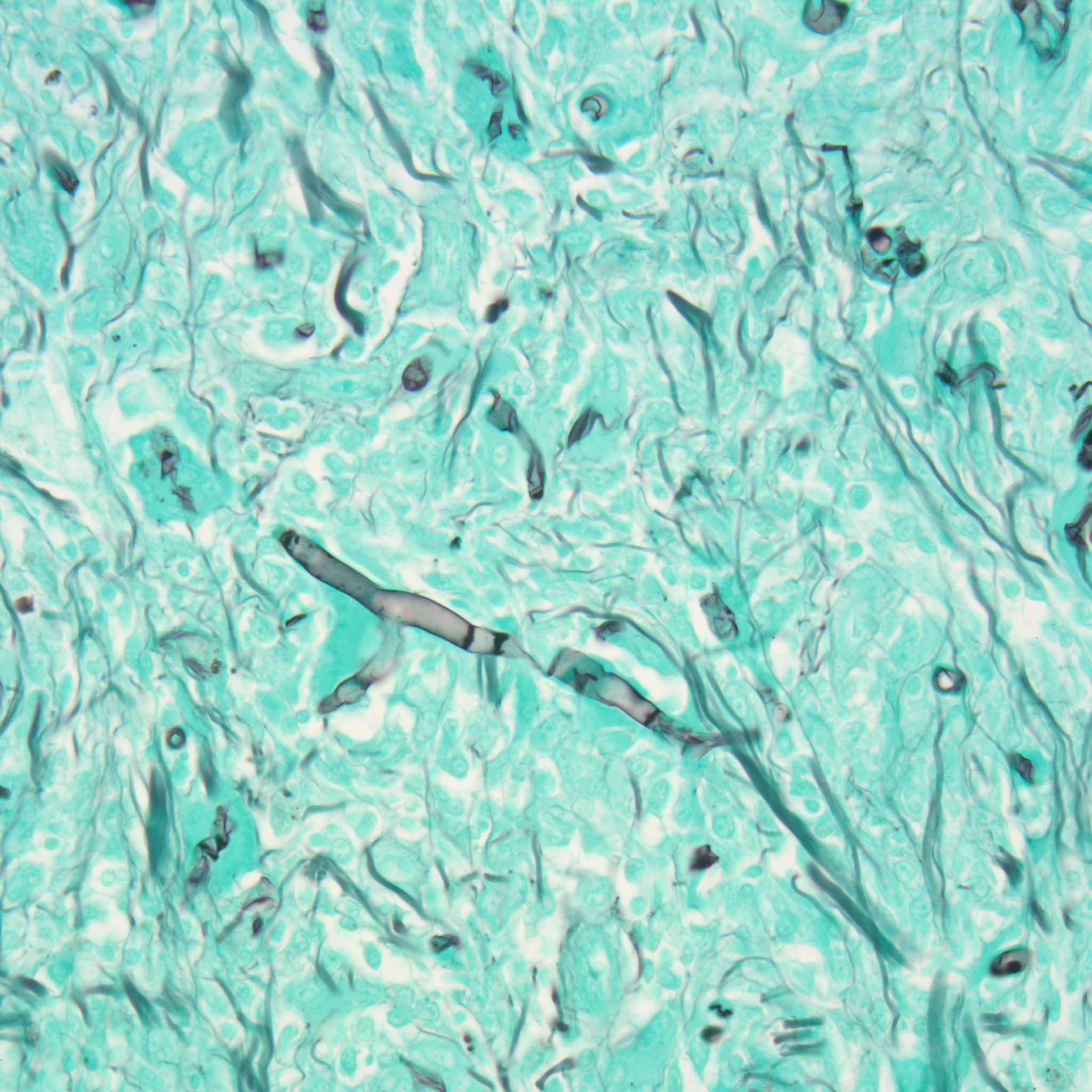
Oomycetes are aquatic dimorphic water molds, and include the genera Lagenidium and Pythium, as well as a number of significant plant pathogens, such as Phytophthora infestans, the organism that caused the Irish potato famine in the mid 19th century.1 Pythium causes two syndromes; the first is cutaneous, which is more common in horses and produces spongy masses that ulcerate and become necrotic5, while the second is gastrointestinal. Hyphae are GMS-positive but PAS-negative, as they do not produce chitin.
Lagenidium produces similar lesions and has a similar geographic distribution, which makes differentiation of pythiosis and lagenidiosis difficult. However, unlike the fairly wide host range of Pythium, Lagenidium appears to be only a pathogen of dogs. Lesions tend to be on the limbs, mammary, and inguinal areas5, with spread to regional lymph nodes. Lagenidium also has a predisposition for invasion of great vessels, with one study finding 70% of cases had vascular invasion.3 Histologic lesions typically are pyogranulomatous with significant numbers of eosinophils.
Treatment of these cases is difficult, and typically consists of surgical resection, chemotherapy, and supportive care.7 Prognosis is guarded and medical therapy is difficult, as one of the main antimycotic targets, ergosterol, is missing in oomycetes.2
Contributing Institution:
University of Florida
College of Veterinary Medicine
Veterinary Diagnostic Laboratory
P.O. Box 100123
Gainesville, FL 32610
http://labs.vetmed.ufl.edu
JPC Diagnosis:
Rectum, anus, perianal tissue: Perianal dermatitis and cellulitis, granulomatous, chronic, focally extensive, severe, with numerous intra- and extracellular hyphae.
JPC Comment:
The contributor provides an excellent review of both of pythiosis and lagenidiosis.
Both agents share similar clinical signs and characteristics within cytologic and histologic sections, including large diameter, non-parallel, rarely septate hyphae associated with eosinophilic granulomatous to pyogranulomatous inflammation.6 Although PCR assay or ribosomal DNA sequencing should be used for definitive differentiation, an important histologic feature that may help to differentiate lagenidiosis from pythiosis is hyphal diameter in tissue. Lagenidum spp. is typically larger and more variable (7 to 25 μm) compared to P. insidiosum (2 to 7 μm). This feature is repeatable when cultured, with Lagenidium sp. (25 to 40 μm) compared to Pythium sp. (4 to 10 μm).6
Participants discussed an additional differential diagnosis of mucormycosis (formerly known as zygomycosis), caused by etiologies within the order Mucorales such as Rhizipus, Mucor, Lichtheima (Absidia), and Saksenaea. As with Pythium and Lagenidium¸ these etiologies are histologically indistinguishable and definitive identification requires culture, PCR, or immunohistochemistry.5
Lagenidium giganteum 's transient historical use as a biopesticide for mosquito control stems from the ability of its biflagellate zoospores to selectively attach and penetrate the cuticle of mosquito larvae and proliferate within, killing the host in less than three days. Under ideal conditions the mycelia differentiate into asexual zoospores to amplify the initial infection but can also remain dormant for days to years until conditions again favor mosquito breeding and germination of spores. Recycling following a single application has been documented for up to 8-10 years, which made its use as a biopesticide an attractive option.4
L. giganteum was registered as a biologic control agent with the US Environmental Health Protection Agency in 1995 under the trade name Laginx, but was later deregistered at the manufacturer 's request in 2011. A subsequent 2013-2014 phylogenic study of 21 mammalian Lagendium strains found 11 could not be differentiated from strains the US Environmental Protection Agency had approved for biological control of mosquitoes; these strains were later unregistered and are no longer available. Although the phylogenically indistinguishable biological control and mammalian strains both grew well at 25⁰C, only the mammalian strains grew well at 37⁰C.9
References:
1. Fry WE, Grünwald NJ: Introduction to Oomycetes. In: The Plant Health Instructor. 2010
2. Grooters AM. Pythiosis, lagenidiosis, and zygomycosis in small animals. Vet Clin North Am Small Anim Pract. 2003;33: 695-720, v.
3. Grooters AM, Hodgin EC, Bauer RW, Detrisac CJ, Znajda NR, Thomas RC. Clinicopathologic findings associated with Lagenidium sp. infection in 6 dogs: initial description of an emerging oomycosis. J Vet Intern Med. 2003;17: 637-646.
4. Kerwin JL. Oomycetes: Lagenidium giganteum. J Am Mosq Control Assoc. 2007;23(2 Suppl):50-57.
5. Mauldin EA, Peters-Kennedy J. Integumentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer 's Pathology of Domestic Animals. Vol 1. 6th ed. St. Louis, MO: Elsevier; 2016:657-660.
6. Rajeev S, Ilha MR, Harrison JM, et al. Pathology in practice. Lagenidium infection. J Am Vet Med Assoc. 2012;241(4):447-449.
7. Schmiedt CW, Stratton-Phelps M, Torres BT, et al. Treatment of intestinal pythiosis in a dog with a combination of marginal excision, chemotherapy, and immunotherapy. J Am Vet Med Assoc. 2012;241: 358-363.
8. Spies CFJ, Grooters AM, Levesque CA, et al. Molecular phylogeny and taxonomy of Lagenidium-like oomycetes pathogenic to mammals. Fungal Biol. 2016;120: 931-947.
9. Vilela R, Taylor JW, Walker ED, Mendoza L. Lagenidium giganteum pathogenicity in mammals. Emerg Infect Dis. 2015;21: 290-297.