CASE 1: A19-490A (4153162-00)
Signalment:
Adult, male, brown anole (Anolis sagrei)
History:
Multiple, free ranging brown anoles in western, central Florida, United States were observed to have multiple raised, disfiguring subcutaneous masses and facial swellings. Over the course of several months, six affected individuals were collected and submitted for post-mortem examination. The submitted photographs, photomicrographs, and histologic sections are from one of these animals. The findings were similar across all six individuals.
Gross Pathology:
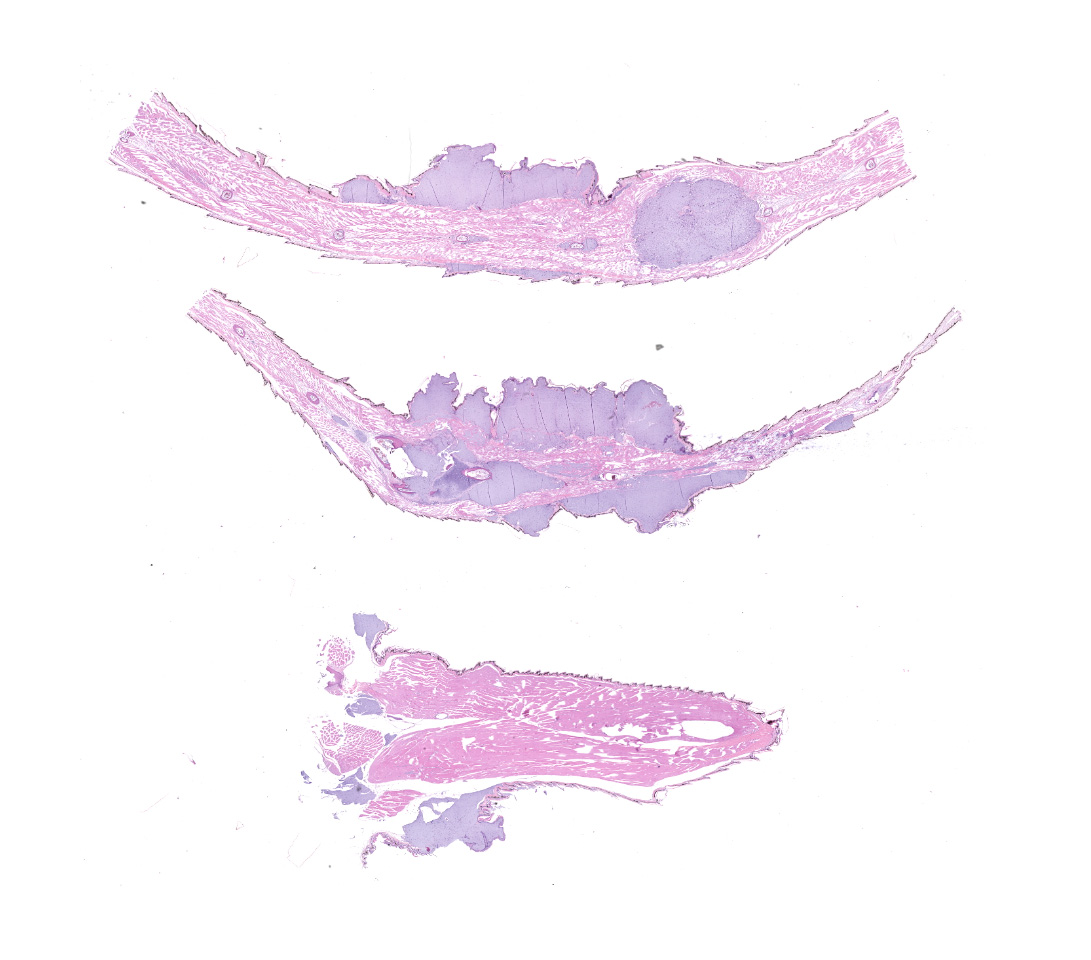
Along the head, trunk, dorsal pelvic region, hindlimbs, and tail, there are tens of multifocal to coalescing, subcutaneous, fluctuant masses, which range from approximately 2 x 2 x 2 mm to 6 x 6 x 4 mm. A few of the masses are superficially ulcerated and one on the ventrolateral aspect of the thoracic body wall is deeply ulcerated. On cut section, the masses are white, opaque, and gelatinous. There is also an ill-defined swelling surrounding the caudoventral aspect of the left mandible, which extends to the left tympanum. The gross internal examination is unremarkable.
The submitted slides include sections from the masses on the tail and one of the hindlimbs.
Laboratory results:
PCR (16S and 16S-23S IGS): 100% sequence identity to a novel Enterococcus sp. reported in reptiles from Christmas Island
Microscopic description:
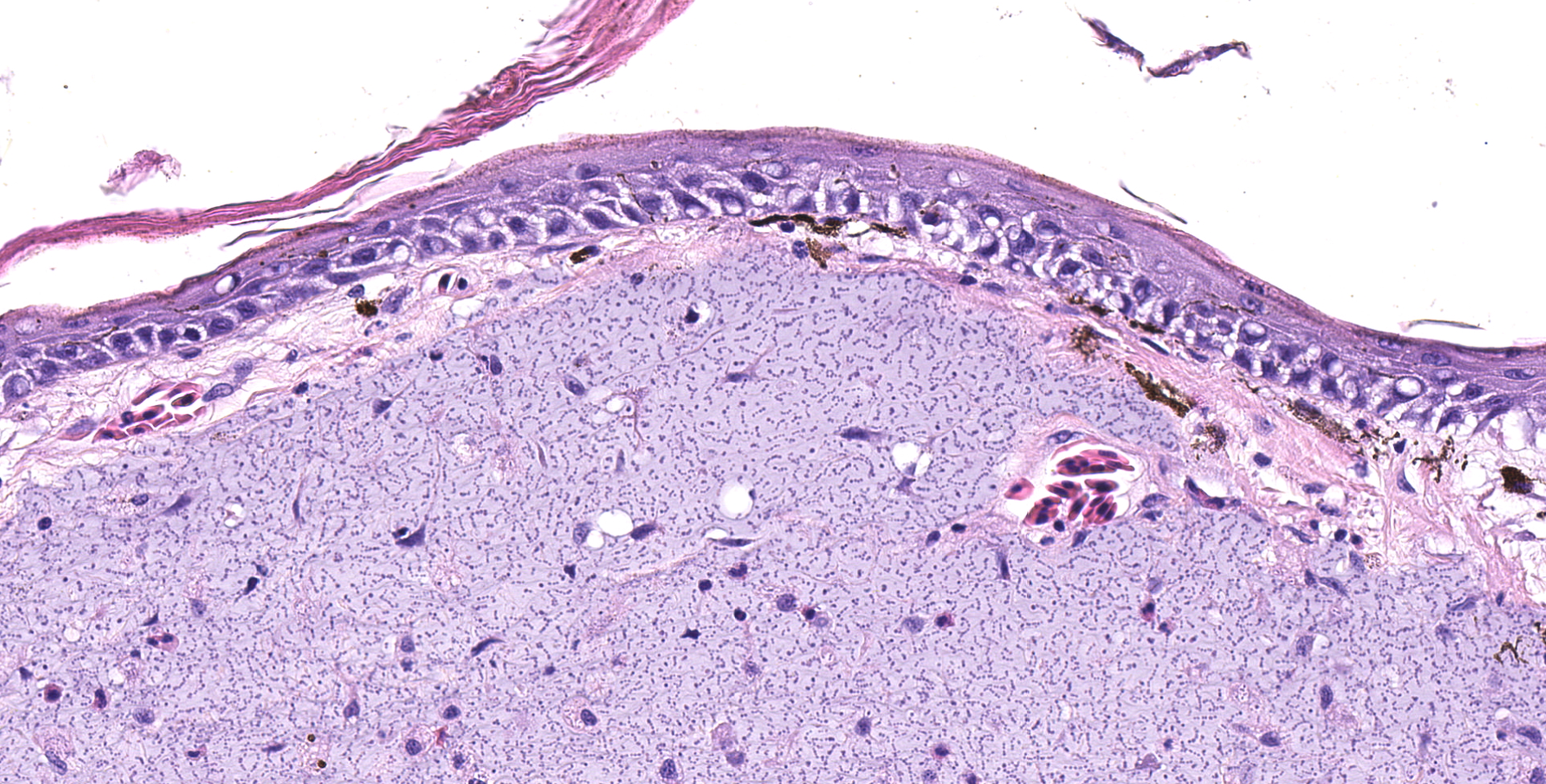
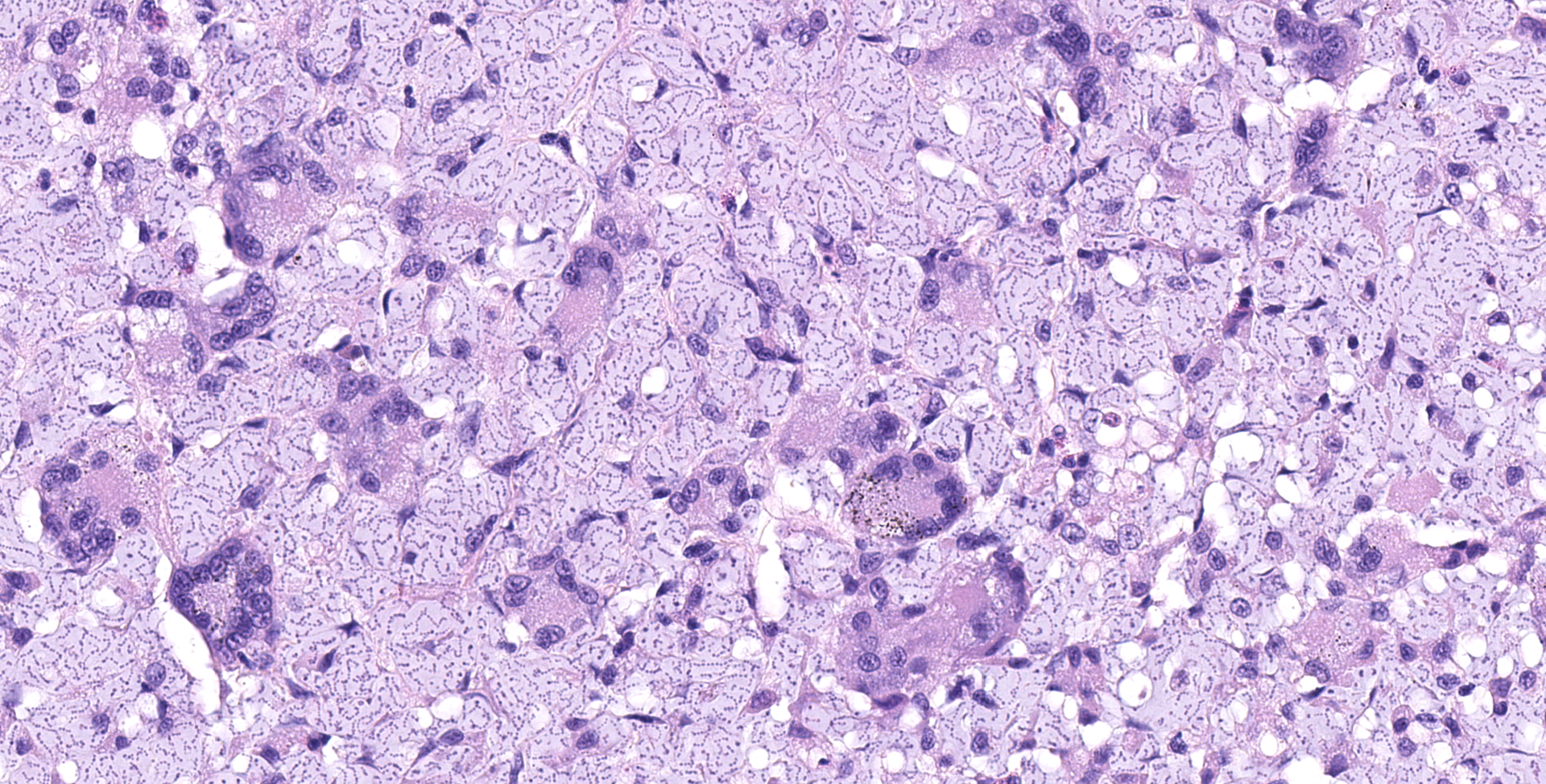
Multifocally elevating the epidermis and infiltrating and expanding the dermis, subcutis, skeletal muscle, and vertebral bone, there are several large, mass-like aggregates of numerous coccoid bacteria. The bacteria are less than 1 micrometer in diameter, and they form distinct, linear chains, which are embedded and surrounded by homogeneous, lightly basophilic material. Admixed with the bacteria and sometimes forming a thin rim around the aggregates, there are low to moderate numbers of scattered macrophages, multinucleated giant cells, lymphocytes, and fewer granulocytes. The epidermis overlying the masses ranges from mildly hyperplastic to multifocally eroded or ulcerated. Areas of ulceration are often covered by a moderately thick crust containing many degenerating granulocytes, sloughed keratin debris, and aggregates of the previously described coccoid bacteria. Skeletal muscle fibers adjacent to the bacterial aggregates sometimes exhibit degenerative changes characterized by myofiber swelling, alterations in staining properties ranging from pallor to hypereosinophilia, fragmentation of the sarcoplasm, and/or nuclear condensation. Aggregates of bacteria multifocally invade and destroy the cortices of vertebral bones, sometimes extending into the spinal canal and wrapping around the spinal cord. Infrequently, a few small aggregates of bacteria with few inflammatory cells are also present within the marrow spaces of vertebrae. The remaining adjacent bone is often irregularly scalloped and exhibits several lightly basophilic reversal lines that parallel the natural margin of the bone. The white matter of the spinal cord contains many empty, dilated axon sheaths, which infrequently contain low numbers of macrophages (digestion chambers). In some, but not all sections, low numbers of nematode larvae are scattered throughout the skeletal muscle, within small cyst-like spaces that are surrounded by a thin rim of epithelioid macrophages and lymphocytes. The nematodes are approximately 80-100 micrometers in diameter, and they have a moderately thick, eosinophilic tegument. They have coelomyarian, polymyarian musculature and prominent lateral cords. There is a central digestive tract lined by several cuboidal to columnar epithelial cells.
Contributor's morphologic diagnosis:
- Dermatitis, myositis, and osteomyelitis, granulomatous and granulocytic, multifocal to coalescing, chronic, marked, with aggregates of chain-forming, coccoid bacteria and associated myofiber degeneration, bony remodeling, and axonal degeneration
- Myositis, granulomatous, multifocal, chronic, mild, with intralesional nematode larvae
Contributor's comment:
Both the gross and histologic lesions observed in this group of brown anoles exhibited striking similarities to those recently reported in several species of lizards from Christmas Island, an Australian province in the Indian ocean.7 Given the morphologic similarities, PCR was performed on a sample obtained from one of the cutaneous masses, and the resultant gene products revealed 100% sequence identity to the novel Enterococcus sp. identified in the lizards from Christmas Island. In both our group of anoles and in all species from Christmas Island (including three species of geckos and one species of skink), the infection was characterized by widespread, multi-organ involvement in which nearly all tissues were multifocally infiltrated and/or partially effaced by similar aggregates of the morphologically distinct bacteria.7 Involvement of the head with destruction of mandibular and maxillary bones was a consistent and prominent finding in both groups of lizards.
Rare historical reports of morphologically similar infections have also been documented in several other species of lizards from Malaysia and Germany, although genomic sequencing was not performed in these cases.5,10 Despite classification of these organisms as streptococcal species based on morphologic features, the similarity of the histologic lesions and bacterial morphology to the more recent cases suggest that the bacteria may have represented either the same species or a closely related species of Enterococcus. In previously reported experimental inoculations, the disease was characterized by high morbidity and mortality and by a slow progression, often taking several months for external lesions to develop and for natural death to ensue.10 The mechanism of bacterial spread is currently unknown, although the frequency of lesions on the head and jaws has led to speculation that fighting and bite wounds may be involved.10
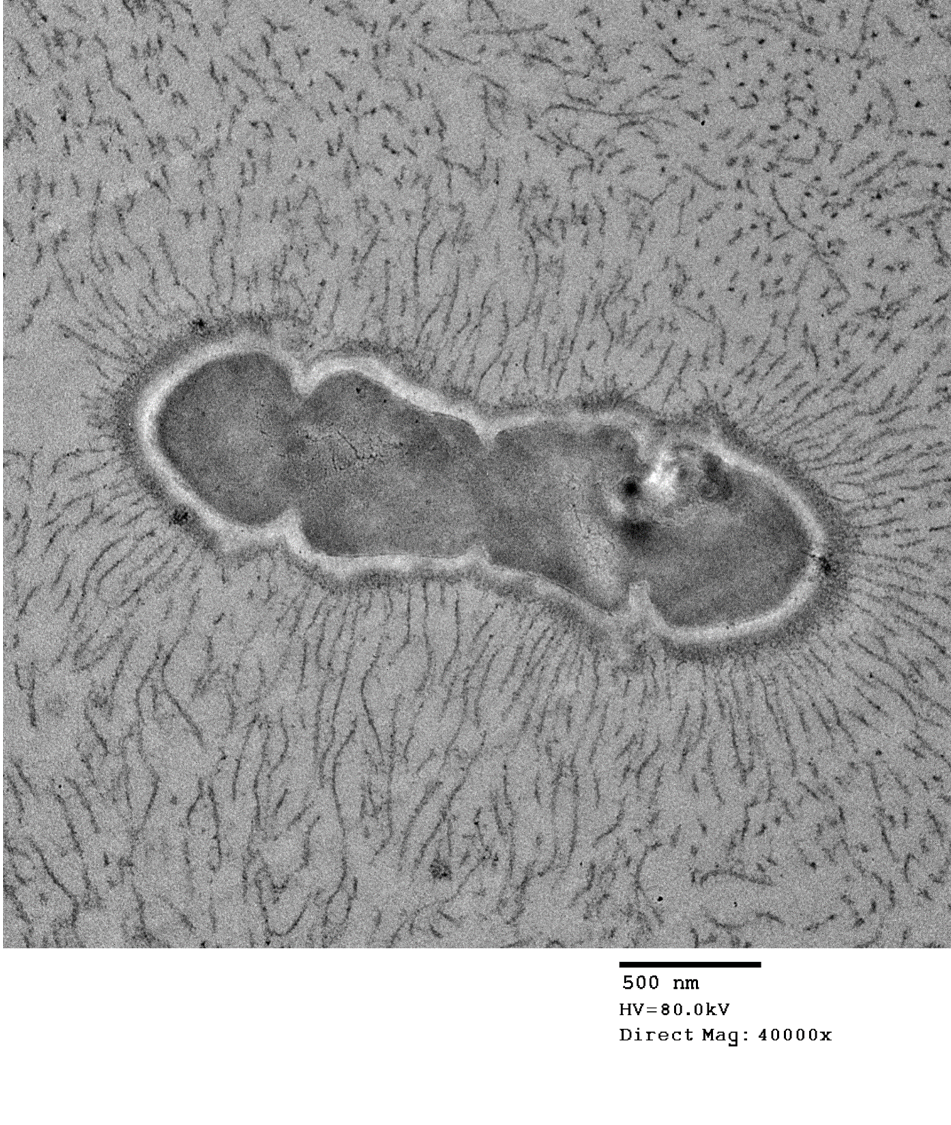
The bacteria exhibit distinctive morphologic features and unique staining properties that make them easy to recognize in cytologic or histologic specimens. The organisms form distinct chains and are embedded within a lightly basophilic matrix material. They are gram-positive, although in our experience, the matrix material can interfere with stain uptake, and diastase digestion prior to Gram staining yielded more consistent results. Uniquely, the organisms are also PAS positive, with PAS staining concentrating in a thin rim along the surface of the bacterium. On electron microscopy, the bacteria have a moderately thick capsule and numerous thin, linear structures that project from the surface into the surrounding matrix material, compatible with fimbriae or pili. Despite the markedly high number of bacteria present within lesions, accompanying inflammation is characteristically mild or sometimes moderate. Presumptively, inflammation may be mitigated by the presence of the thick matrix material in which these organisms are embedded, especially as matrix deposition, the formation of pili, and subsequent biofilm production is a commonly reported contributor to pathogenicity in other species of Enterococcus.1,7
In general, Enterococcus species are ubiquitous organisms that represent common gastrointestinal commensals of many species, including humans, domestic mammals, and birds.1,4 Most frequently, pathogenicity is associated with opportunistic infections, and multi-drug resistance is commonly reported in both humans and veterinary species.1,4 Enterococcus sp. are fastidious organisms and often require specific media conditions for successful growth in culture.7
Although not present in all submitted slides, many of the slides included one or more sections of intramuscular nematode larvae within thin-walled granulomas. These organisms were considered incidental and likely unrelated to the systemic Enterococcus sp. infection.
Contributing Institution:
University of Florida College of Veterinary Medicine
Department of Comparative, Diagnostic, and Population Medicine
JPC diagnosis:
1. Limbs and tail, dermis, skeletal muscle, bone: Large colonies of cocci with mild lymphoplasmacytic and histiocytic dermatitis, brown anole, lizard.
2. Limb: Granulomas, multiple, with nematode larvae.
JPC comment:
During conference discussion, it was noted that some sections and slides may not have contained nematode larvae within granulomas. However, a short discussion of anatomic and morphologic features of nematodes and their larvae took place.
The contributor concisely describes Enterococcus infections across several species. Much of the body of knowledge about Enterococcus species has been built from research on E. faecalis and E. faecium, pathogens that produce disease in humans. Features that contribute to virulence and pathogenicity include aggregation substance (an adhesin), a capsule, the cell wall, pili, cytolysin, secretion of extracellular superoxide, gelatinase, iron acquisition, enterococcal surface protein (esp), and a variety of microbial surface components recognizing adhesive matrix molecules (MSCRAMMs).8
Enterococcus faecium, though a normal commensal of the human gastrointestinal tract, is a frequent cause of septicemia in hospitalized patients. Using transcriptome profiling (RNA-seq) and high-throughput transposon mutant library sequencing (Tn-seq) to evaluate a vancomycin-resistant clinical isolate, specific genes were identified that allowed for survival and exponential growth in human serum. Several genes involved in de novo nucleotide biosynthesis (pyrK_2, pyrF, purD, and purH) and carbohydrate uptake (manZ_3, manY_2, ptsL) conferred the greatest virulence, and were critical to growth in serum.9
In poultry, Enterococcus cecorum infection has been documented extensively, worldwide, since its first description in 1983. While this disease is notable for causing skeletal disease in broiler and broiler breeder chickens, the most striking feature is paralysis caused by an inflammatory mass that develops in the spinal column. This usually results in the bird sitting with both legs extended cranially and is considered a classic symptom.3
Other reported infections in the brown anole also include Ranavirus (OIE reportable in amphibians but not reptiles), reovirus, and fungi of the Chrysosporium anamorph of Nannizziopsis vriesii-complex (CANV-complex).6
A feature unique to some species of Lacertilia that has spurred research involves fracture planes and regrowth of lost tails. Though it lacks normal structure and is paler, muscle is regenerated, along with a cartilaginous tube in place of true vertebra.6 The key feature that allows regeneration is the blastema formation at the site of detachment, an aggregate of proliferating cells under a new, scar-free, epithelial layer. The cells of the blastema differentiate into all regenerated tissue types, though is likely lineage-restricted to one germ layer. While there are variations in regeneration of axolotls, salamanders, telosts, and lizards, current evidence indicates that ependymal cells of the spinal cord are most critical to the coordinated growth and differentiation of the blastema. With lizards the closest living relatives to mammals that are capable of scar-free healing, they have become the animal model for regenerative research in humans.2
References:
1. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10: 266-278.
2. Gilbert EAB, Delorme SL, Vickaryous MK. The regeneration blastema of lizards: an amniote model of the study of appendage replacement. Regeneration. 2015;2(2):45-53.
3. Jung A, Chen LR, Suyemoto MM, Barnes HJ, Borst LB. A review of Enterococcus cecorum infection in poultry. Avian Diseases. 2018;62:261-271.
4. Kataoka Y, Umino Y, Ochi H, Harada K, Sawada T. Antimicrobial susceptibility of enterococcal species isolated from antibiotic-treated dogs and cats. J Vet Med Sci. 2014;76: 1399-1402.
5. McNamara TS, Gardiner C, Harris RK, Hadfield TL, Behler JL. Streptococcal Bacteremia in Two Singapore House Geckos (Gekko monarchus). Journal of Zoo and Wildlife Medicine. 1994;25: 161-166.
6. Origgi FC. Lacertilia. In: Terio KA, McAloose D, St. Leger J, ed. Pathology of Wildlife and Zoo Animals. San Diego, CA: Elsevier. 2018:871-887.
7. Rose K, Agius J, Hall J, et al. Emergent multisystemic Enterococcus infection threatens endangered Christmas Island reptile populations. PLoS One. 2017;12: e0181240.
8. Stewart Gc. Streptococcus and Enterococcus. In: McVey DS, Kennedy M, Shengappa MM eds. Veterinary Microbiology. Ames, IA: Wiley-Blackwell. 2013:199-201.
9. Zhang X, de Matt V, Prieto AMG, et al. RNA-seq and Tn-seq reveal fitness determinants of vancomycin-resistant Enterococcus faecium during growth in human serum. BMC Genomics. 2017;18:893.
10. Zwart P: Streptokokkensepsis mit Hautwucherungen bei Eidechsen. International Conference of Zoo Animal Diseases, 1972