CASE II: C-23638-17 (JPC 4119604)
Signalment:
5 month old, intact, female, Nova Scotia duck tolling retriever, Canis lupus familiaris
History:
The owners report that until the morning of presentation the dog had been an apparently normal active puppy, although they thought she always appeared to drink and urinate excessively. On the morning in question, she refused her breakfast, went outside to urinate and had a semi-solid bowel movement, then came back in the house and laid down on her bed. The owners looked in on her 30 minutes later and she was not moving and did not appear to be breathing. The owners rushed her to their vet where she was pronounced dead on arrival. The owners consented to a postmortem.
Gross Pathology:
The puppy was thin, having small visceral and subcutaneous fat stores. A few streaks of hemorrhage were noted on the abdominal surface of the diaphragm. The urinary bladder was small and empty. The caudodorsal lung lobes were red, heavy and wet, while cranioventral areas were pale pink and pliable.
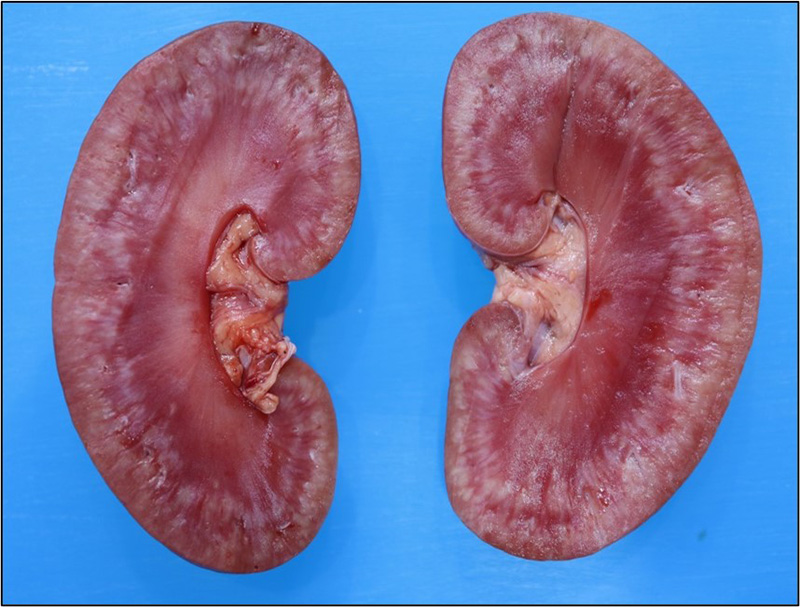
Otherwise, significant postmortem findings were restricted to the kidneys. Both were mildly enlarged, slightly pale and the capsules were mildly, diffusely adhered to the cortices. When sectioned, much of the cortical parenchyma is effaced by poorly
defined, multifocal to coalescing, pale, white-tan, streaks which extend into the peripheral medulla.
Laboratory Results:
A scant growth of E. coli (2 colonies) was isolated from the kidney.
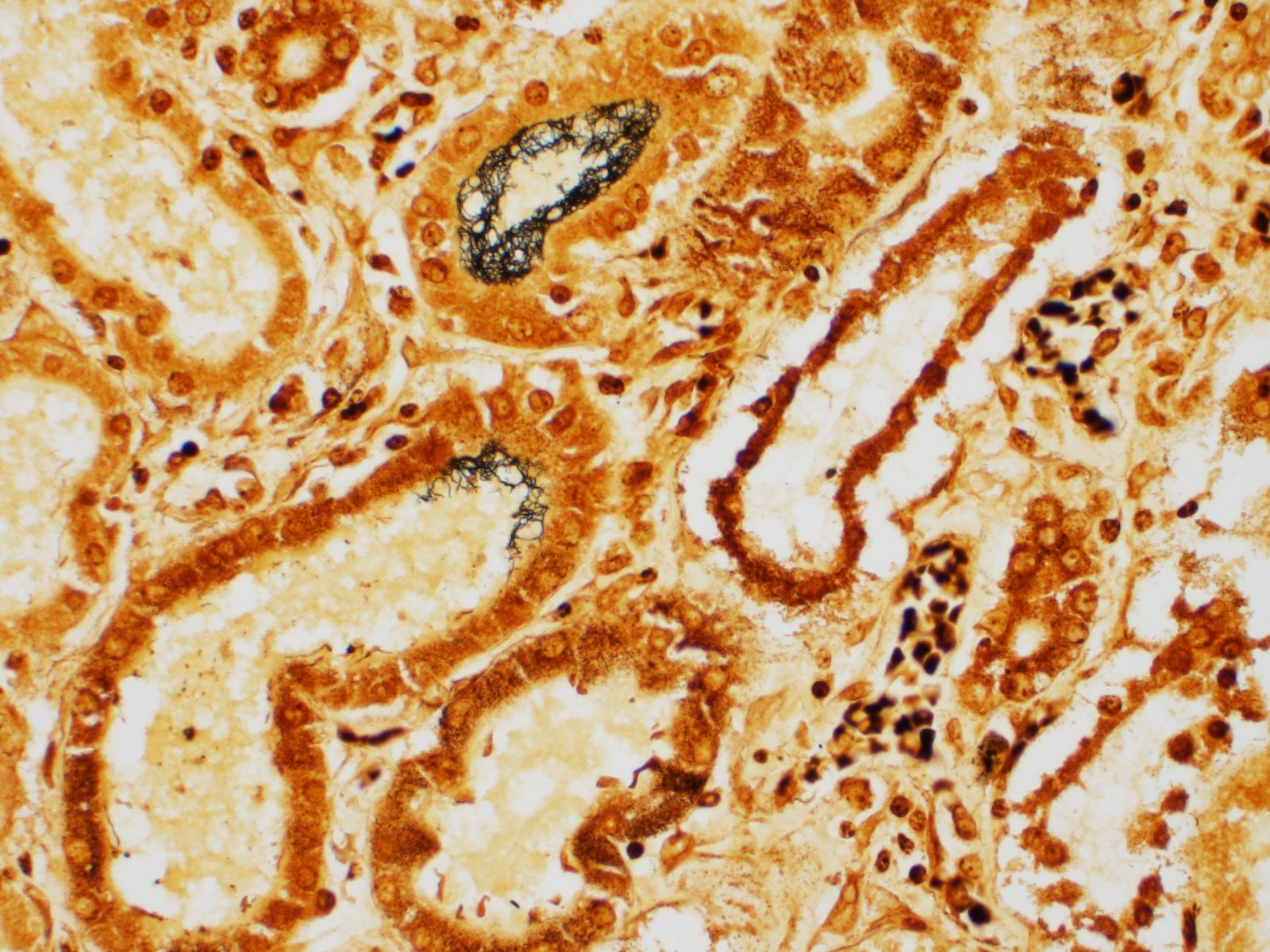
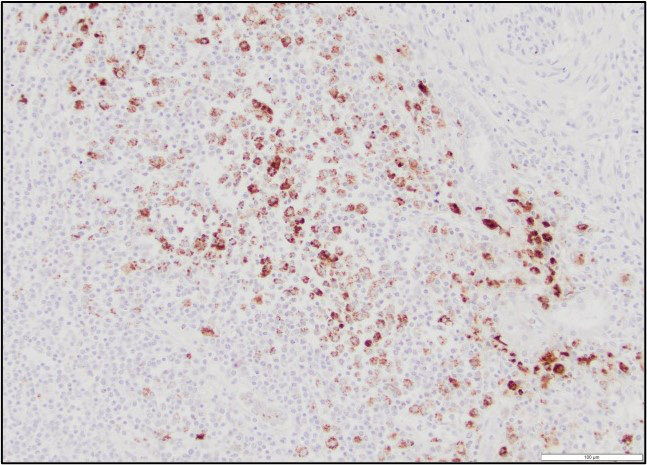
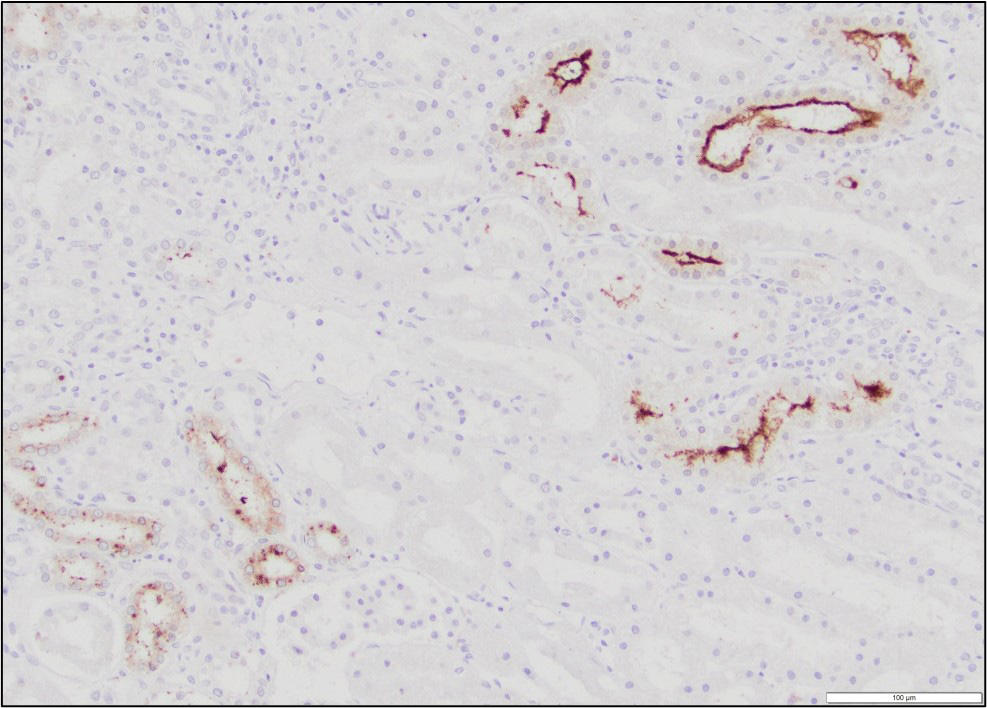
Immunohistochemistry performed on sections of kidney revealed moderate, multifocal, staining for Leptospiral antigens within areas of inflammation. The staining was cytoplasmic in inflammatory cells (largely macrophages) and was also occasionally noted at the luminal surface of tubular epithelial cells.
Microscopic Description:
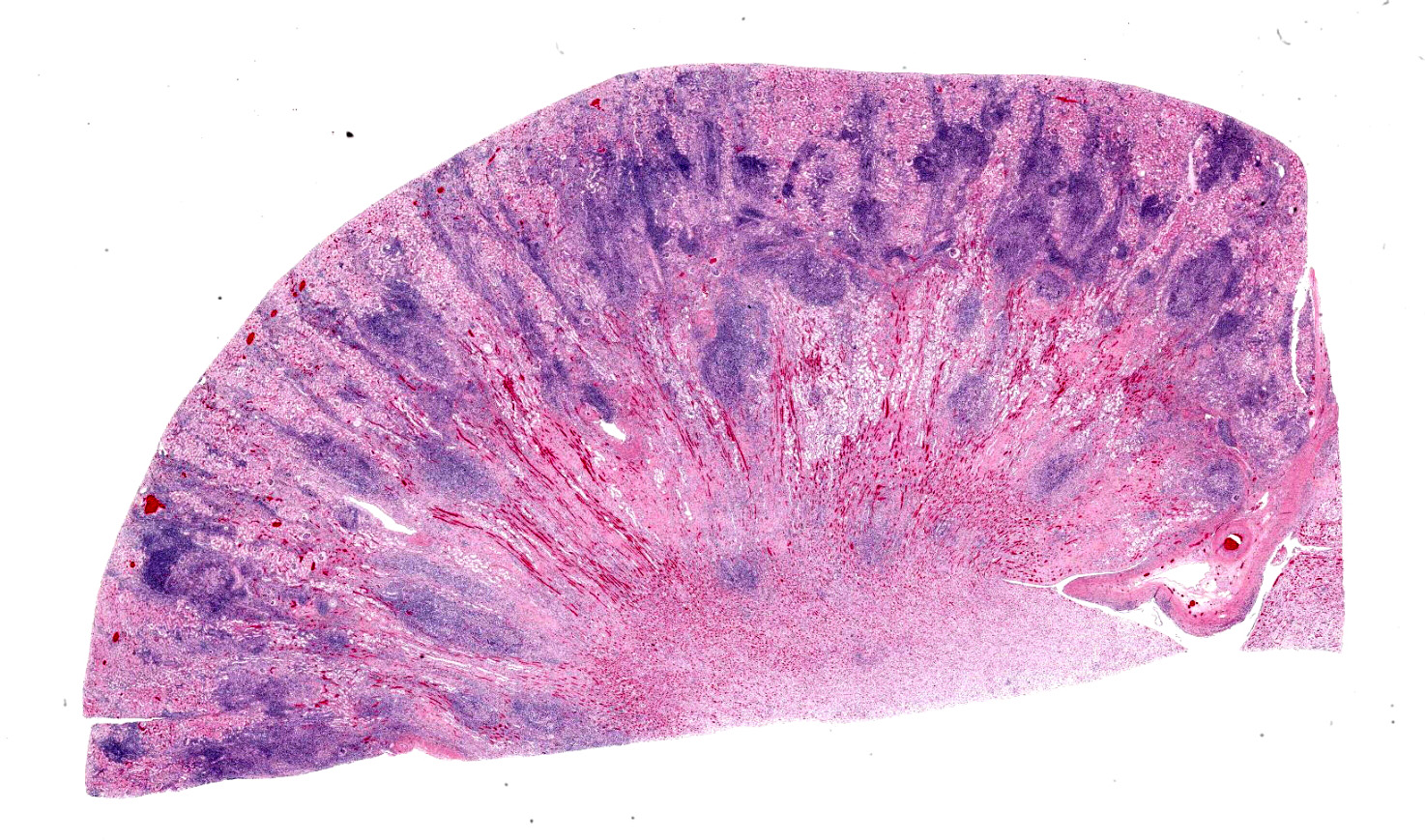
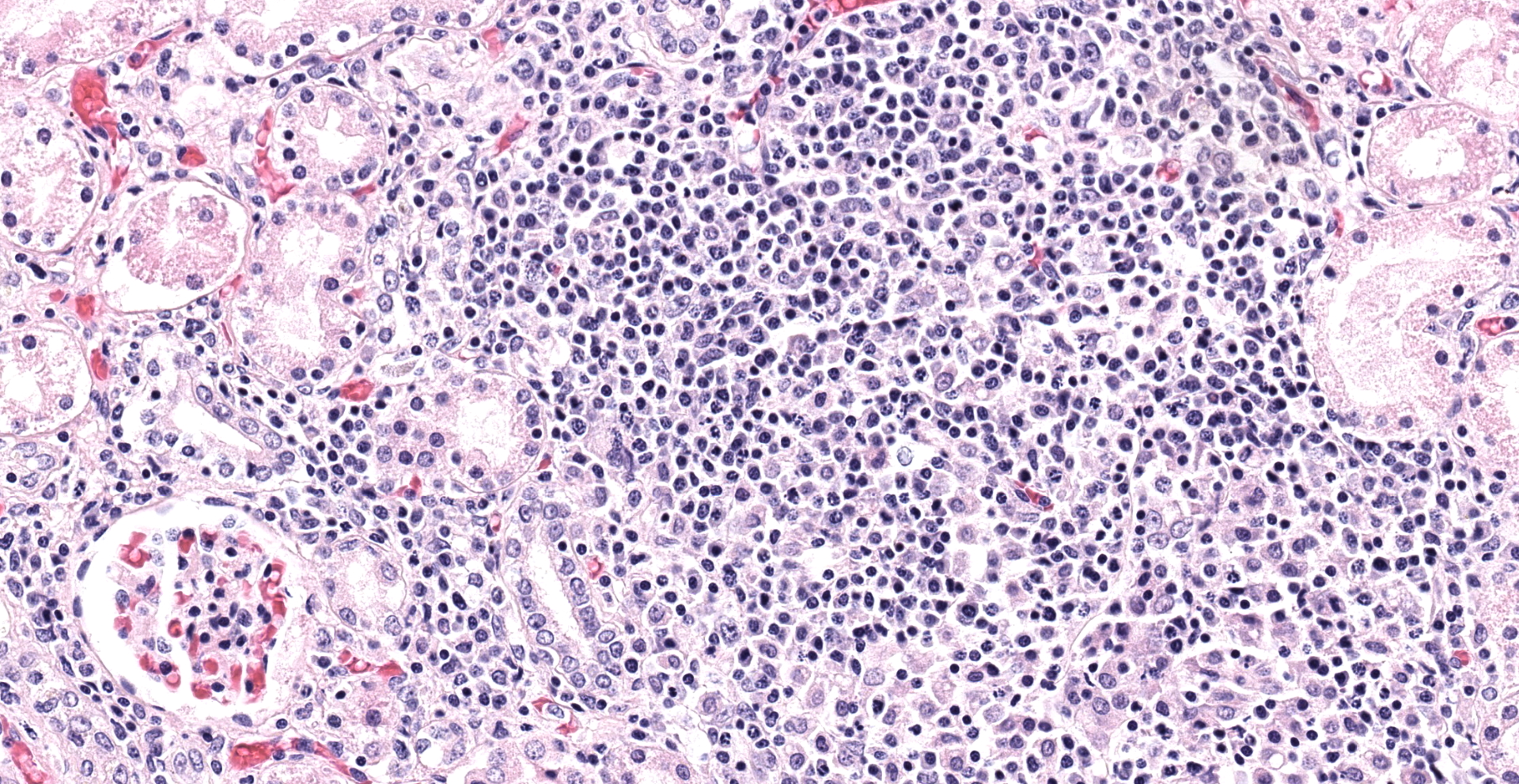
The white-tan areas noted grossly in the renal cortices and peripheral medullary areas represents numerous, multifocal to coalescing, areas where the interstitium is expanded and the parenchyma is replaced by dense infiltrates of lymphocytes admixed with fewer plasma cells, and sometimes central aggregates of pale epithelioid macrophages. Sparse cell debris is scattered amongst these infiltrates which are associated with a mild to moderate increase in collagenous stroma that often extends into the adjacent medulla. Scattered cortical and medullary tubules within the intervening parenchyma contain small amounts of proteinaceous fluid and, rarely, sparse cell debris. These tubules are often lined by epithelial cells which appear slightly floccular with unapparent nucleoli and/or the epithelium has partially sloughed. Gram, PAS and acid fast staining does not reveal infectious agents. Warthin-Starry staining reveals finely stippled black material (putative bacteria or possibly cell debris) and occasional fine, thin bacilli-like structures within macrophages and loose in areas of inflammation, and rarely within epithelial cells lining adjacent intact tubules.
Contributor's Morphologic Diagnoses:
Kidney: Severe, bilateral, chronic, multifocal to coalescing, lymphohistiocytic, interstitial nephritis
Contributor's Comment:
The sudden death of this puppy was attributed to renal failure caused by chronic interstitial nephritis due to leptospiral infection. The diagnosis was confirmed by immunohistochemistry; leptospiral antigens were demonstrated within macrophages in areas of inflammation, as well as within the lumen of occasional adjacent renal tubules. Unfortunately, further serotyping/genotyping of the bacteria was not performed.
Leptospirosis is a zoonotic spirochetal bacterial infection with worldwide distribution that affects many animal species. The taxonomy of Leptospires is complicated, confusing, and evolving. Leptospires were originally subdivided via culture and immunologic reactivity into 2 species; L. interrogans sensu lato (pathogenic strains) and L. biflexa sensu lato (all saprophytic strains from the environment). Within these 2 species Leptospires were then classified
into approximately 25 serogroups which have been further subdivided to more than 250 serovars of Leptospira.3 Serogroups include serovars that share common antigens resulting in cross-reactions with antibody detection methods, while serovars are identified by distinct antibody reactivity to a variety of carbohydrate moieties in the outer lipopolysaccharide membrane.3 Unfortunately, due to the nature of this type of antibody testing, it has limited value in trying to definitively identify these organisms by species because different species and serovars often share common antigenic determinants and cross-reactions are extremely common. That is, antigenically similar serovars may belong to different Leptospira species. Serogrouping and serovar typing, although not perfect, has had practical uses in epidemiologic studies and it has been thought that the protection induced by current vaccines is restricted to the serogroup used for their production.7 Serotyping is increasingly being replaced by restriction enzyme analysis of chromosomal DNA which has resulted in the identification of approximately 20 genomospecies of Leptospira.3
In general, serovars of leptospires are carried and spread in the environment by specific maintenance hosts to which that serovar is well adapted. The maintenance host does not typically develop clinical disease but carries the bacteria in renal proximal tubules, or, in some hosts (cattle, sheep, horses), the genital tract. These animals shed bacteria in the urine for several months or throughout life. Rodents, raccoons, skunks, dogs, cattle, sheep, and horses have all been identified as maintenance hosts for one or several of the 10 serogroups/serovars of Leptospires that are associated with clinical disease in veterinary medicine. Clinical disease is generally seen when an "incidental" host becomes infected. Transmission is typically via contact with contaminated urine, or less commonly, through contact with contaminated placental/fetal tissues or by venereal transmission.1 Leptospires do not replicate outside of the maintenance host but they can survive in warm, moist, environmental conditions (such as stagnant water and water logged soil) for weeks to months. Because of this, the incidence of disease is often seasonal with clinical disease most commonly occurring in the fall in temperate climates ("fall fever"), in the winter in tropical climates, or following periods of heavy rainfall or flooding.1
In dogs, several serovars of Leptospira are associated with clinical disease. Historically, canicola and icterohaemorrhagiae are best recognized as a cause of disease and mortality. In the 1970's, a bivalent vaccine for dogs targeting these serovars became available, and now, disease in dogs due to these serovars is rare. However, in recent years, northeastern North America, including areas within the Maritime provinces of Canada6, has seen an apparent resurgence of leptospirosis in urban dogs. Serovars identified in these dogs include grippotyphosa, pomona, autumnalis, bratislava and other serovars typically seen in wildlife. This resurgence may be due to a combination of factors including, vaccination (current vaccines do not offer protection against these serovars), changes in climate (increased periods of heavy rain and flooding, etc) and increased contact with wild maintenance hosts in urban environments (skunks, rodents, raccoons, etc).7
The severity of clinical signs induced by leptospiral infection varies widely from subclinical disease to peracute or chronic manifestations of disease and depends on factors such as age, immune status or the host's ability to contain infection, infective dose, serovar type and virulence. Leptospires can penetrate intact mucous membranes of the mouth, nose, eyes and abraded, water-softened skin. The organisms then enter systemic circulation and quickly begin to replicate. Severe peracute infection resulting in septicemia has been reported in puppies (usually infected with icterohemorrhagiae) and death may occur in a few hours to 2-3 days.1,7 Endothelial damage and hemorrhage are commonly seen in these animals. In most infected dogs this stage of infection is subclinical or associated with vague clinical signs. Organisms then disperse and replicate in many tissues, most commonly the kidney and liver. Acute death has been reported in dogs in this stage of infection. At postmortem, these dogs may have areas of hepatic necrosis and microscopic lesions consisting of dissociation of hepatic cords, canaliculi plugged with bile, acute hepatocellular degeneration and early regeneration, and organisms may be noted with silver stains in sinusoids and within hepatocytes.
Subacute to chronic leptospirosis is the form of disease most often seen in dogs with leptospirosis. This typically manifests as renal insufficiency resulting from subacute to chronic interstitial nephritis as the bacteria localize and replicate in the kidney. The severity and progression of disease may be quite variable. In acute renal lesions, tubular degeneration and regeneration predominates. As lesions progress, tubular lesions resolve, interstitial infiltrates of lymphocytes and plasma cells increase and then often generally decrease, as interstitial fibrosis becomes gradually more prominent. The organisms may be noted in areas of inflammation and within cortical tubules with Warthin-Starry stains in subacute lesions but generally become less numerous with chronicity.1
Leptospirosis is of zoonotic concern. Humans (usually pet owners, veterinary staff, etc.), may be infected via contaminated urine, or rarely via bite wounds, so special care and hygienic precautions should be taken when handling infected dogs.3
Contributing Institution:
Atlantic Veterinary College, University of Prince Edward Island
JPC
Diagnosis: Kidney: Nephritis, tubulointerstitial,
lymphoplasmacytic and histiocytic, chronic, multifocal to coalescing, marked,
with tubular degeneration, necrosis, and loss, and interstitial fibrosis.
JPC Comment:
The contributor provides an excellent review of leptopirosis, the most common zoonotic infection in the world.7 German physician Adolf Weil first described leptospirosis in 1886 after recognizing a "new" infectious disease characterized by splenomegaly, jaundice, and nephritis which was subsequently known as "Weil's disease" in humans. Inada et al. at Kyushu University first successfully isolated Leptospira spp. nearly three decades later during a 1914-1915 outbreak of Weil's disease in Japanese coal miners. Although Weil typically receives credit for first recognizing the distinct clinical disease, historical records indicate knowledge of this condition since ancient times based on its seasonality, occupational exposure, and duration of symptoms with names such as "rice-harvest jaundice" in ancient China as and "7-day fever" and "autumn fever" in Japan.4
As noted by the contributor, certain leptospiral serovars are associated with specific maintenance hosts that are often asymptomatic, such as Leptospira borgpetersenii serovar Hardjo and Leptospira interrogans serovar Hardjo in cattle, Leptospira interrogans serovar Canicola in dogs, Leptospira interrogans serovar Pomona in pigs, and Leptospira interrogans serovar Copenhageni in rats. In contrast, "incidental" hosts typically develop clinical disease.4
The pathogenesis of subclinical chronic tubulointerstital nephritis in maintenance hosts has been the subject of interest. Based on experimental L. interrogans serovar Copenhageni infection in rats, the pathogen disseminates hematogenously to nearly all tissues shortly after infection, followed shortly afterward by its elimination from nearly all tissues, with the exception of the kidneys, which is likely facilitated by circulating anti-leptospiral immunoglobulin (IgM and IgG). However, the kidneys present a permissive environment for the organism's survival and proliferation, and leptospiruria develops within a week of infection. Given the clearance of leptospirosis from nearly every organ with the exception of the kidneys, it is likely that kidneys are not a specific target of Leptospira spp. but rather their survival in this organ, specifically in the renal tubules, is facilitated by an immunologically permissive environment that is exploited by the pathogen.5
A possible explanation for this survival despite the presence of anti-leptospiral IgG in the renal tubules is the absence of complement in the renal tubules. In addition, pathogenic Leptospira genomes encode a group of proteins known as Len (leptospiral endostatin-like) proteins that bind complement regulatory proteins such as plasma factor H, which promote deactivation of the compliment cascade, essentially preventing destruction of the cell. Interestingly Len proteins are not present in non-pathogenic Leptospira, further supporting the hypothesis of their role in immune invasion. In addition, human complement regulator C4BP is also bound by pathogenic Leptospira spp., which provides additional host complement resistance.5
In addition to affecting the kidney and liver, leptospirosis may also cause meningitis, uveitis (suspected etiologic agent of equine recurrent uveitis), abortion and infertility, and pulmonary hemorrhage secondary to previously described vasculitis.2
Key features noted by conference participants in this case included lympho-plasmacytic interstitial nephritis in addition to relatively minimal glomerular changes in regions with minimal inflammation and necrosis. Two additional differentials proposed by the moderator in this case include Leishmania spp. and Borrelia burgdorferi. Both of these entities are also associated with lymphoplasmacytic interstitial nephritis; however, the primary lesion associated with these etiologies in the kidney is glomerulonephritis whereas the glomerulus is not typically affected in cases of leptospirosis.
References:
1. Cianciolo RE, Mohr FC. Leptospirosis. In: Maxie MG, ed. Pathology of Domestic Animals. 6th ed. Vol 2. Elsevier Saunders; 2016:433-438.
2. Goldstein RE. Bacterial diseases. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. Vol 1. 7th ed. St. Louis, MO: Saunders Elsevier; 2010:863-864.
3. Greene CE, Sykes JE, Moore GE, Goldstein RE, Shultz RD. Leptospirosis. In: Greene CE, ed. Infectious Diseases of the dog and cat. 4th ed. Elsevier Saunders; 2012: 431-447.
4. Kim MJ. Historical Review of Leptospirosis in the Korea (1945 - 2015) [published correction appears in Infect Chemother. 2020 Jun;52(2):305-306]. Infect Chemother. 2019;51(3):315-329.
5. Monahan AM, Callanan JJ, Nally JE. Review paper: Host-pathogen interactions in the kidney during chronic leptospirosis. Vet Pathol. 2009;46(5):792-799.
6. Prescott J. Canine leptospirosis in Canada: a veterinarian's perspective. Can Med Assoc J. 2008: 178(4): 397-398.
7. Van de Maele, I, Clause A, Haesebrouck F, Daminet S. Leptospirosis in dogs: a review with emphasis on clinical aspects. Vet Rec. 2008: 163:409-413.
8. Wang S, Stobart Gallagher MA, Dunn N. Leptospirosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 13, 2021.