Signalment:
Gross Description:
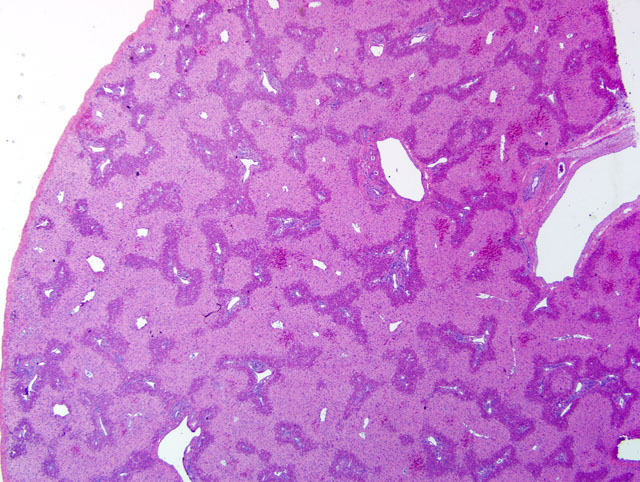
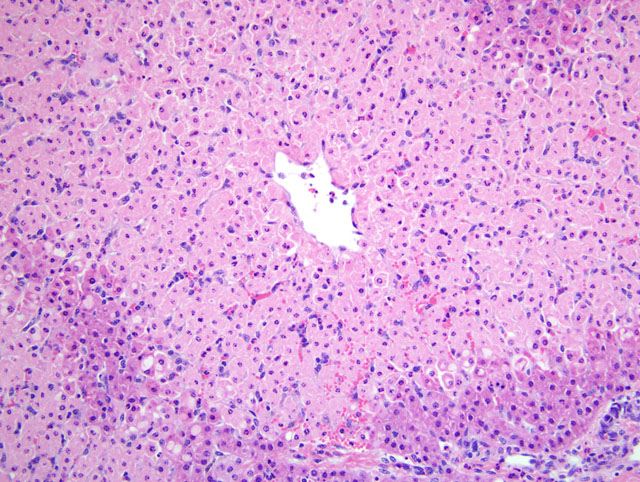
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Carboxyatractyloside and other atractylosides are inhibitors of cellular oxidative phosphorylation, and act specifically by binding to and inhibiting ADP/ATP carriers, leading to ATP depletion and subsequent mitochondrial dysfunction, ion pump failure, lipid peroxidation, and glutathione depletion resulting in cellular apoptosis and/or necrosis.(4,5) All members of the Xanthium genus seem to produce carboxyatractyloside, and other plants in the families Asteraceae and Compositae may produce the same or similar glycosides causing similar clinical syndromes in susceptible species, including cattle, sheep, swine, and humans.(2,3,5-8)
The common cocklebur, a coarse herbaceous annual, is common throughout much of the United States; it grows to a mature height of 2-5 feet, with an erect, often angled stem, and alternate, triangular or heart-shaped rough leaves. The plant produces hard, prickly, oval fruits or burs approximately 0.75 inches long containing two seeds; these can be found entangled in the coats of livestock and long-haired dogs. The plants are invasive and are often found growing in pastures and meadows (especially those with a history of previous or seasonal flooding), along fencerows, in roadside ditches, along stream and pond banks, in dried out ponds or stock reservoirs, and occasionally in disturbed areas in feedlots.(7)
Cocklebur poisoning is most common in the spring or early summer, associated with the ingestion of germinated seeds and palatable young dicotyledon seedling plants that are high in the toxic principle, carboxyatractyloside; adult plants contain relatively little toxin, other than in seeds or burs. Most poisonings seem to occur in pigs foraging naturally, but the plant is toxic to a wide range of animals, including ruminants, horses, dogs, rats, and humans; most cases of toxicity in these species are caused by incorporation of seedlings or mature plants with seeds into feed rations (hay, haylage, silage, or grain rations).(2,3,6,8) As with the submitted case, there are several reports of poisoning in cattle associated with the presence of mature cocklebur plants and seeds (burs) in hay.(8)
Common clinical signs observed with cocklebur poisoning include anorexia, depression or other behavioral changes including apprehension or excitability, blindness, ataxia, twitching progressing to spasmodic muscle contractions or convulsions, recumbency, opisthotonos, and rapid progression to death. Clinical signs may follow ingestion of the plant by as short a period as several hours in monogastric animals and may be delayed for a day or so in ruminants. Characteristic gross lesions of cocklebur poisoning are not specific, but can include ascites and various effusions, hemorrhages (associated with consumption of clotting factors), hepatic swelling, congestion and mottling, fibrin tags on serosal surfaces of viscera, renal congestion, and gastrointestinal congestion.(2,4,8) Microscopic lesions generally are confined to the liver, with characteristic centrilobular/periacinar (zone 3) to midzonal (zone 2) or rarely panzonal hepatocellular degeneration, necrosis, and apoptosis with congestion and/or hemorrhage; however, lesions also may be observed in the kidney (e.g. tubular epithelial degeneration and necrosis) and brain (e.g. neuronal degeneration/necrosis and cerebral edema) on occasion.(2,4,5,8) Diagnosis of cocklebur poisoning generally requires some combination of the following: 1) evidence of ingestion of cotyledonary seedlings or seeds/burs, 2) appropriate history and clinical signs, 3) characteristic clinical pathology findings, and 4) consistent gross and microscopic lesions. Diagnostic assays that detect the toxic principle in tissues or other biological samples have been or are being developed, but none are routinely or widely available to veterinary diagnosticians.
Treatment of affected animals is generally unrewarding once clinical signs have progressed to the neurological stage, and there is no antidote for the toxic principle; supportive care and therapy aimed at increasing gastrointestinal clearance of ingested plants, decreasing gastrointestinal absorption of the toxin, and treating metabolic and neuromuscular complications all have been shown to be effective on occasion. Prevention of poisoning is more effective than treatment of clinical cases, and can be achieved by elimination of plant populations (e.g. mowing before seed production begins, use of herbicides, limiting access to contaminated pastures and meadows, manual removal of plants from hay fields, etc.).
JPC Diagnosis:
Conference Comment:
The bulk of the discussion during the conference was focused on toxic plants that cause centrilobular to midzonal hepatocellular necrosis. Several of these are summarized in the table below:(1)
| Hepatotoxic Plants Causing Centrilobular Necrosis | |||
|---|---|---|---|
| Plant Family | Plants | Species Affected | Toxic Principle |
| Compositae | Xanthium spp. | Pigs, cattle | Carboxyatractyloside |
| Myoporaceae | Myoporum spp. | Pigs, cattle, sheep, horses | Furanosesquiterpenoid oils (ngaione) |
| Leguminosae | Cassia spp. | Cattle | Unknown |
| Ulmaceae | Trema aspera | Cattle, sheep, goats | Trematoxin |
| Solanaceae | Cestrum parqui | Cattle, sheep | Saponins |
| Zamiaceae Cycadaceae Strangeriaceae | Cattle, sheep, goats, dogs | Methoxymethanol | |
| Fabaceae | Indigofera linnaei | Cattle, dogs | Indospicine |
| Cyanophyceae | Microcystis spp., Aphanizomenon spp. | Cattle, sheep, goats, horses, dogs | Microcystins, others |
As mentioned by the contributor and well-illustrated by this case, the investigation of a suspected plant intoxication often involves linking a number of pieces of evidence, including clinical signs, pathological and clinical pathological findings, examination of the feed and/or environment for the offending plant(s), and ancillary diagnostics, when available. In this case, the use of an investigational assay to detect carboxyatractyloside in rumen contents proved helpful in substantiating the diagnosis.
References:
2. Martin T, Stair EL, Dawson L: Cocklebur poisoning in cattle. J Am Vet Med Assoc 189:562-563, 1986
3. Mendez, MC, dos Santos RC, Riet-Correa F: Intoxication by Xanthium cavanillesii in cattle and sheep in southern Brazil. Vet Human Toxicol 40:144-147, 1998
4. Stalker MJ, Hayes MA: Liver and biliary system: toxic hepatic disease. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, pp. 368-369. Saunders Elsevier, Philadelphia, PA, 2007
5. Stuart, BP, Cole RJ, Gosser HS: Cocklebur (Xanthium strumarium, L. var. strumarium) intoxication in swine: review and redefinition of the toxic principle. Vet Pathol 18:368-383, 1981
6. Turgut M, Alhan CC, Gurgoze M, Kurt A, Dogan Y, Tekatli M, Akpolat N, Aygun D: Carboxyatractyloside poisoning in humans. Ann Tropical Paediatr 25:125-134, 2005
7. Whitson TD, Burrill LC, Dewey SA, Cudney DW, Nelson BE, Lee RD, Parker R, Ball DA, Cudney D, Dewey SA, Elmore CL, Lym RG, Morishita DW, Swan DG, Zollinger RK: Common cocklebur. In: Weeds of the West, ed. Whitson TD, 9th ed., pp. 194-195. Western Society of Weed Science/Western United States Land Grant Universities Cooperative Extension Service/University of Wyoming, Laramie, WY, 2001
8. Witte ST, Osweiler GD, Stahr HM, Mobley G: Cocklebur toxicosis in cattle associated with the consumption of mature Xanthium strumarium. J Vet Diagn Invest 2:263-267, 1990