CONFERENCE 23, CASE 3
Signalmant: 7 years old, female, domestic rabbit (Oryctolagus cuniculus)
History: The animal presented with a swelling of the left buccal region, and a firm nodular mass was identified, described as being in the lip (not obviously attached to the gingiva/bone). The submitting veterinary surgeon performed an incisional biopsy and a single piece of tissue was submitted for histopathologic examination. Following initial diagnosis, further surgery was undertaken to remove the lesion in its entirety, attached to a small areas of surrounding buccal/labial skin.
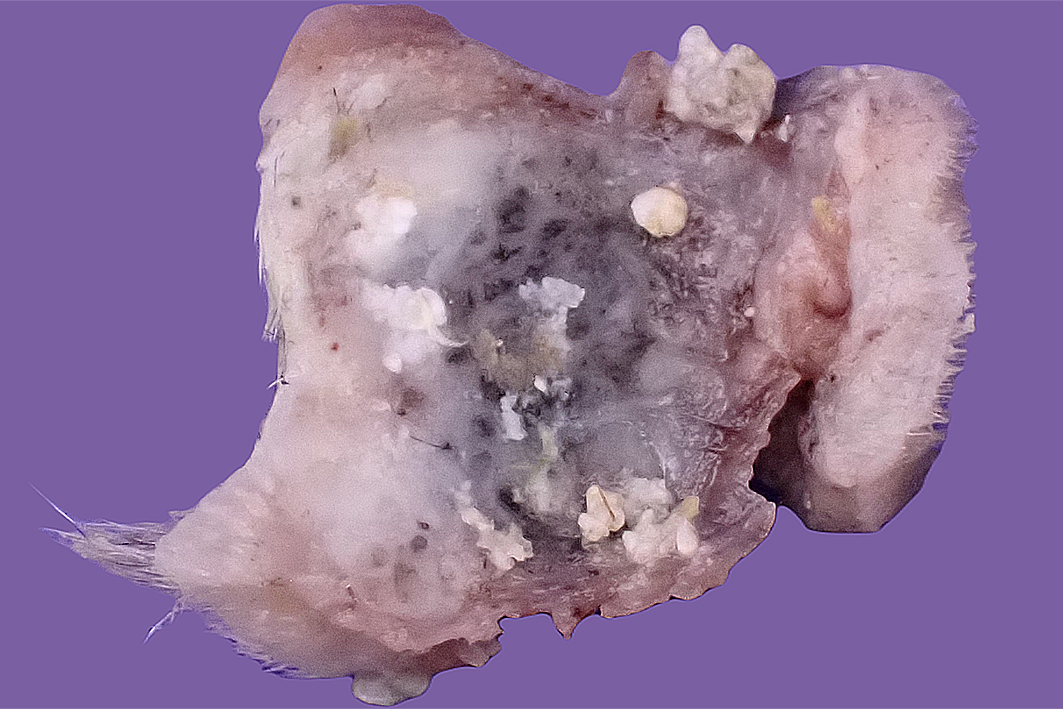
Gross Pathology: The resection specimen was an irregularly ovoid firm nodule (approximately 30 x 25 x 18 mm greatest dimensions) attached to a piece of haired skin (Fig. 1). Incision revealed a relatively well-circumscribed non-encapsulated nodule, with firm to hard, slightly gritty to chalky, grey, cream, and tan mottled cut surfaces (Fig. 2). The lesion required overnight decalcification prior to histological processing.
Laboratory Results:
N/A
Microscopic Description:
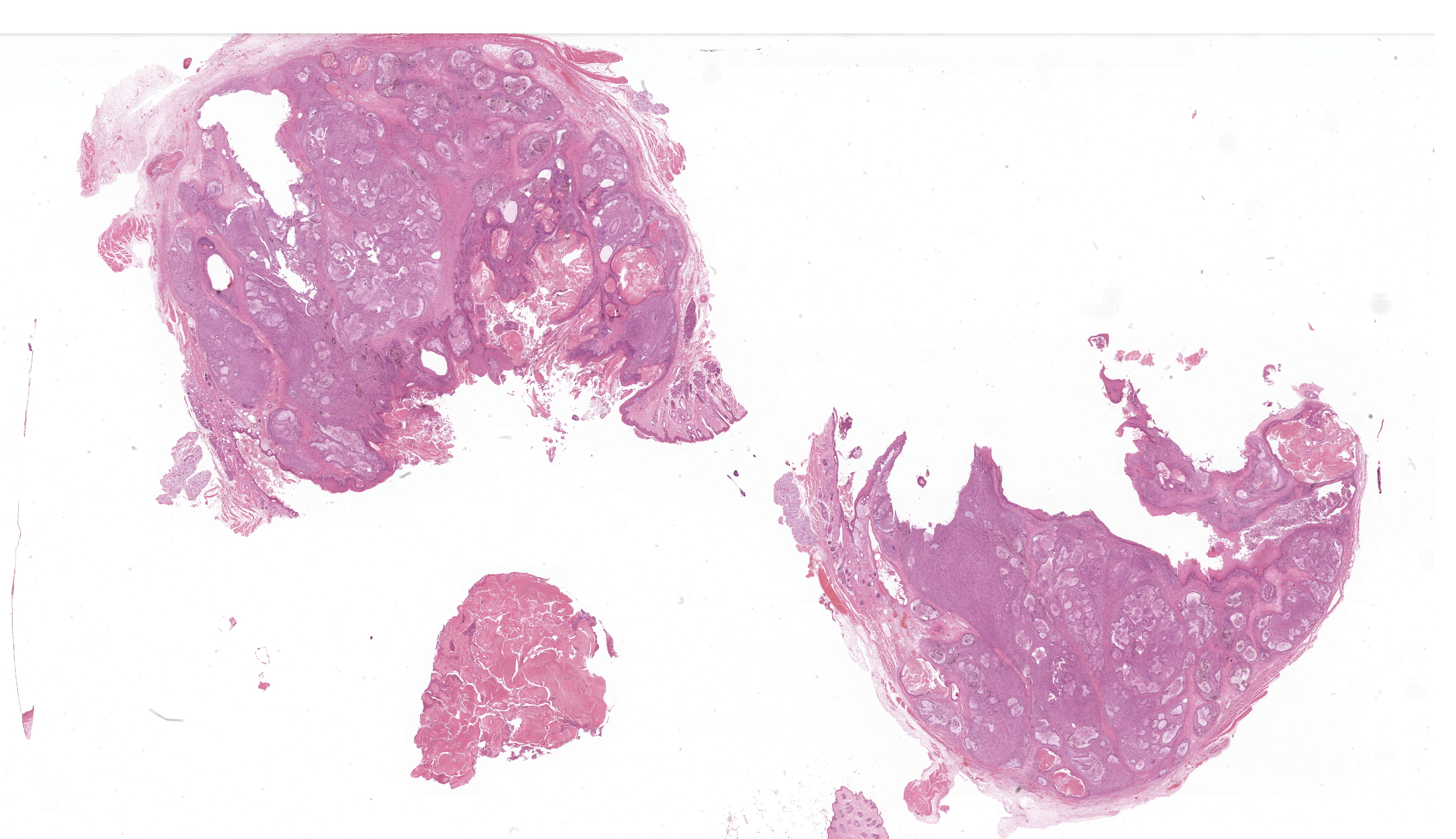
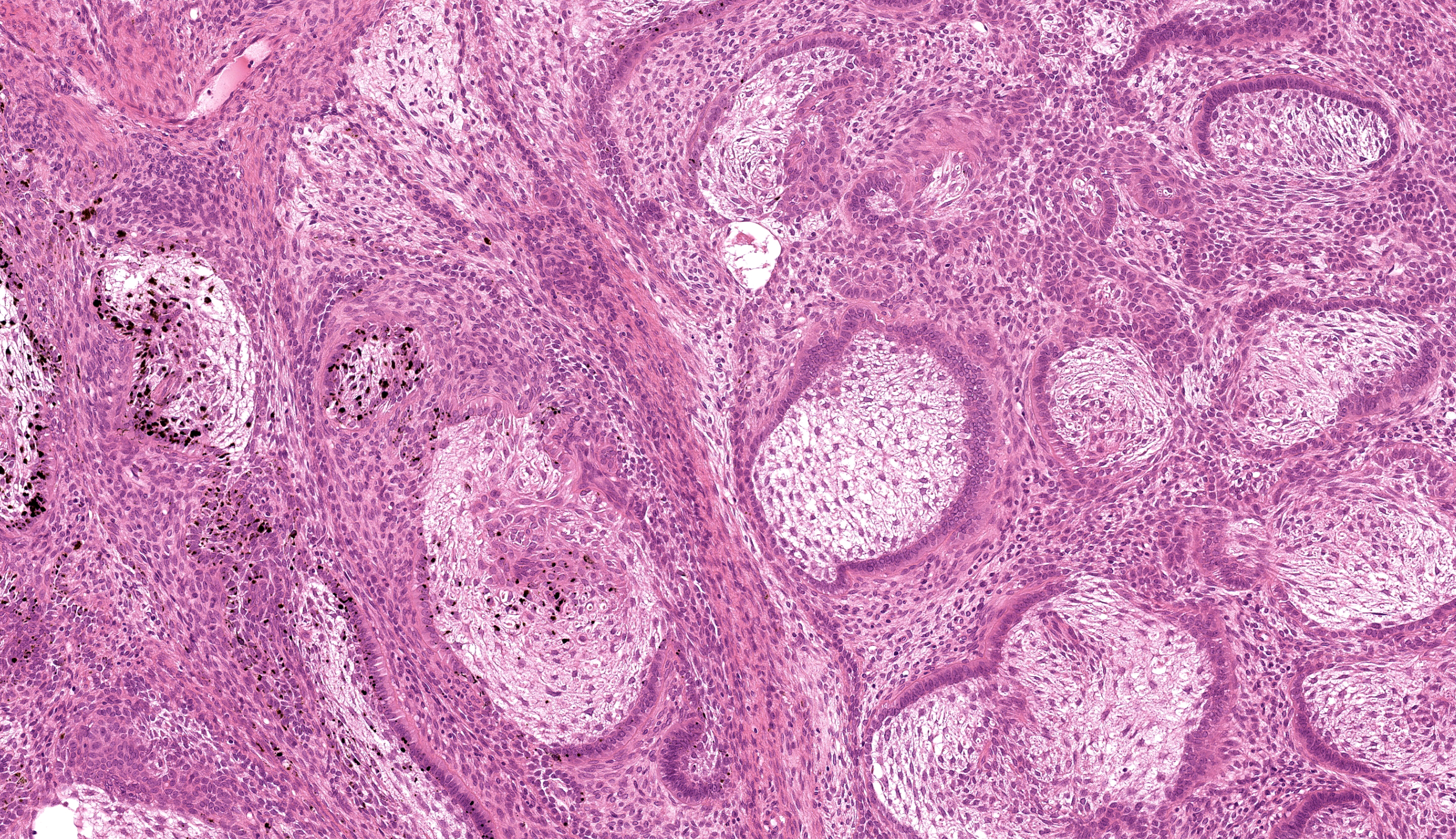
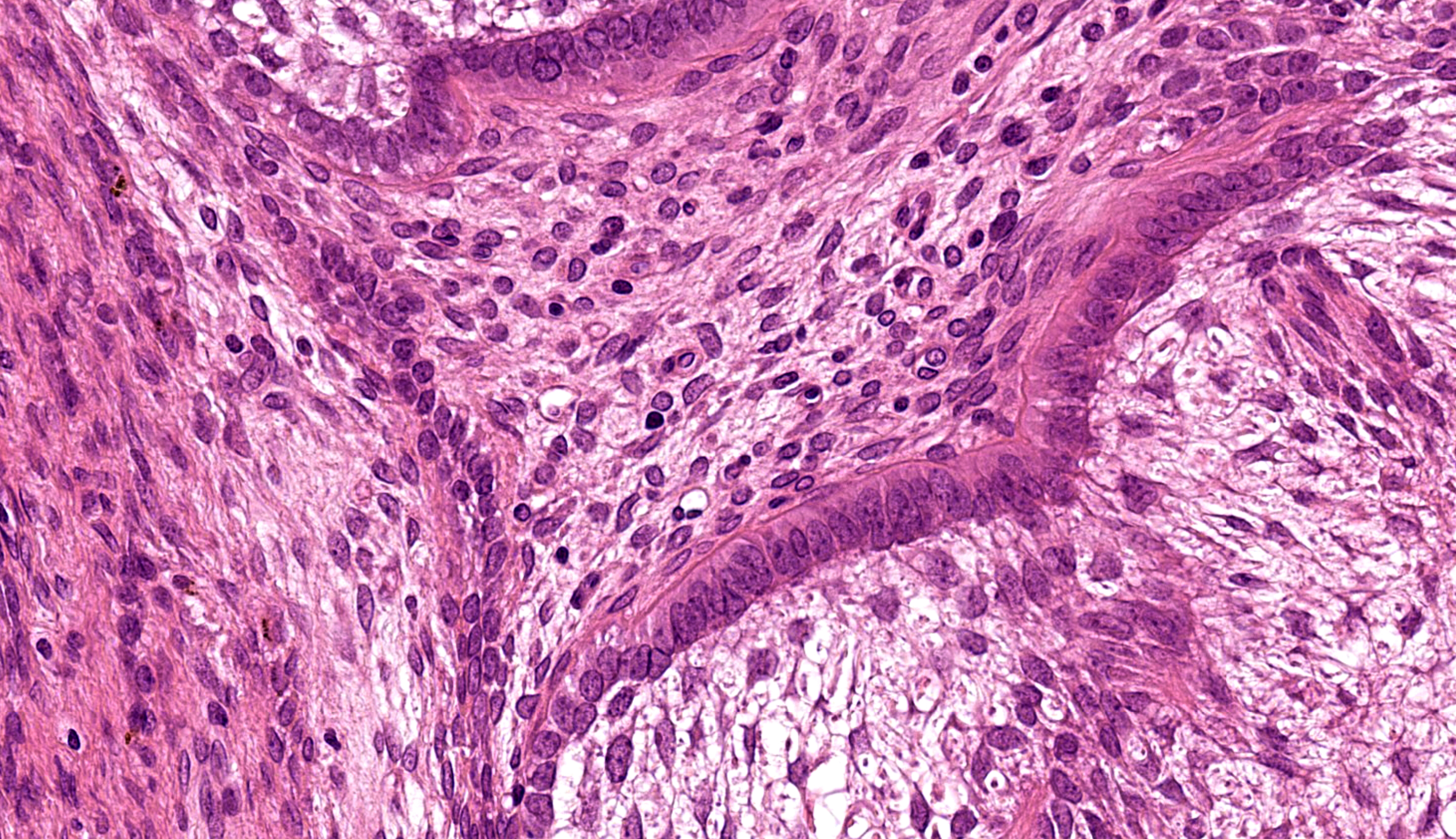
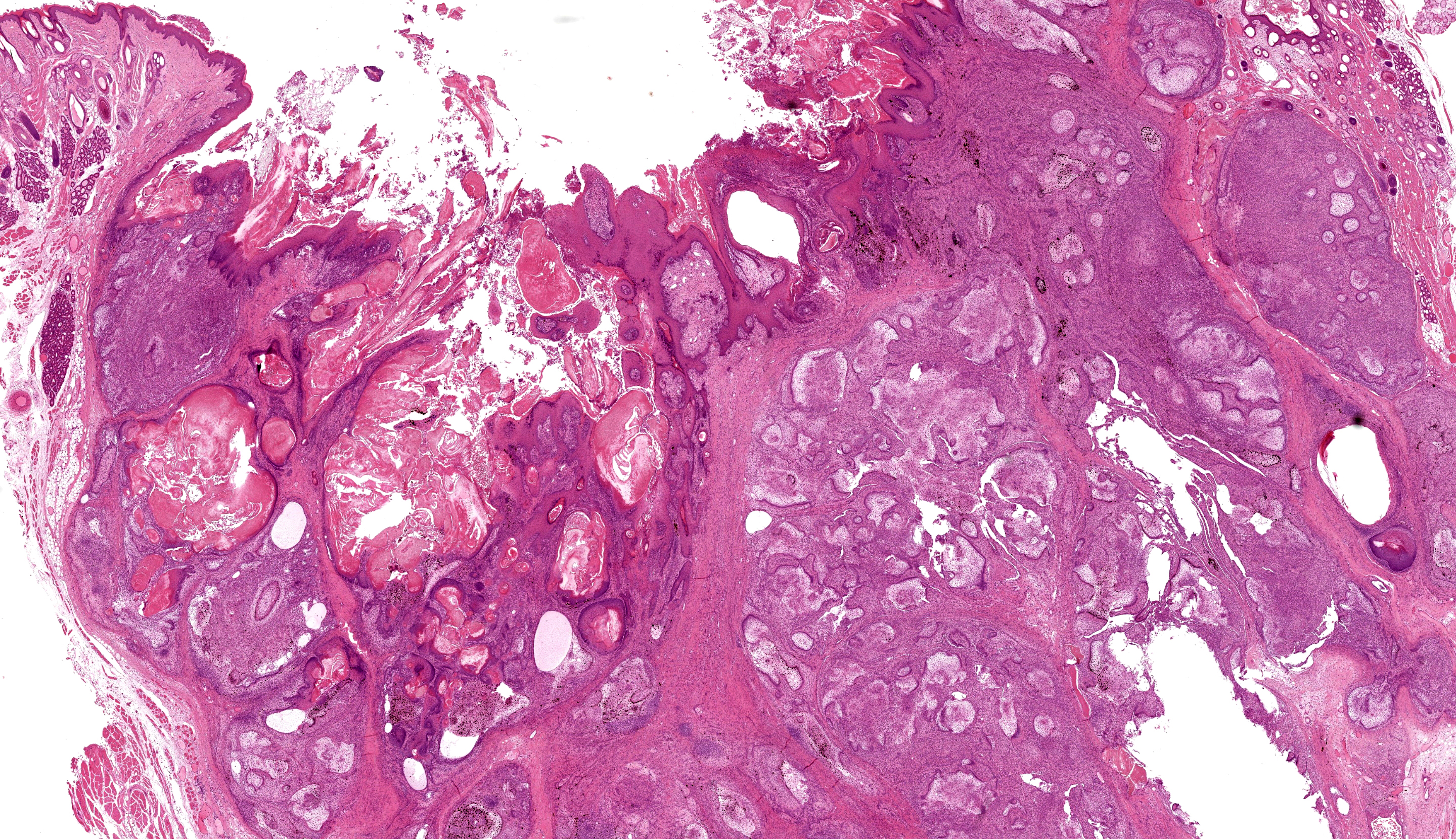
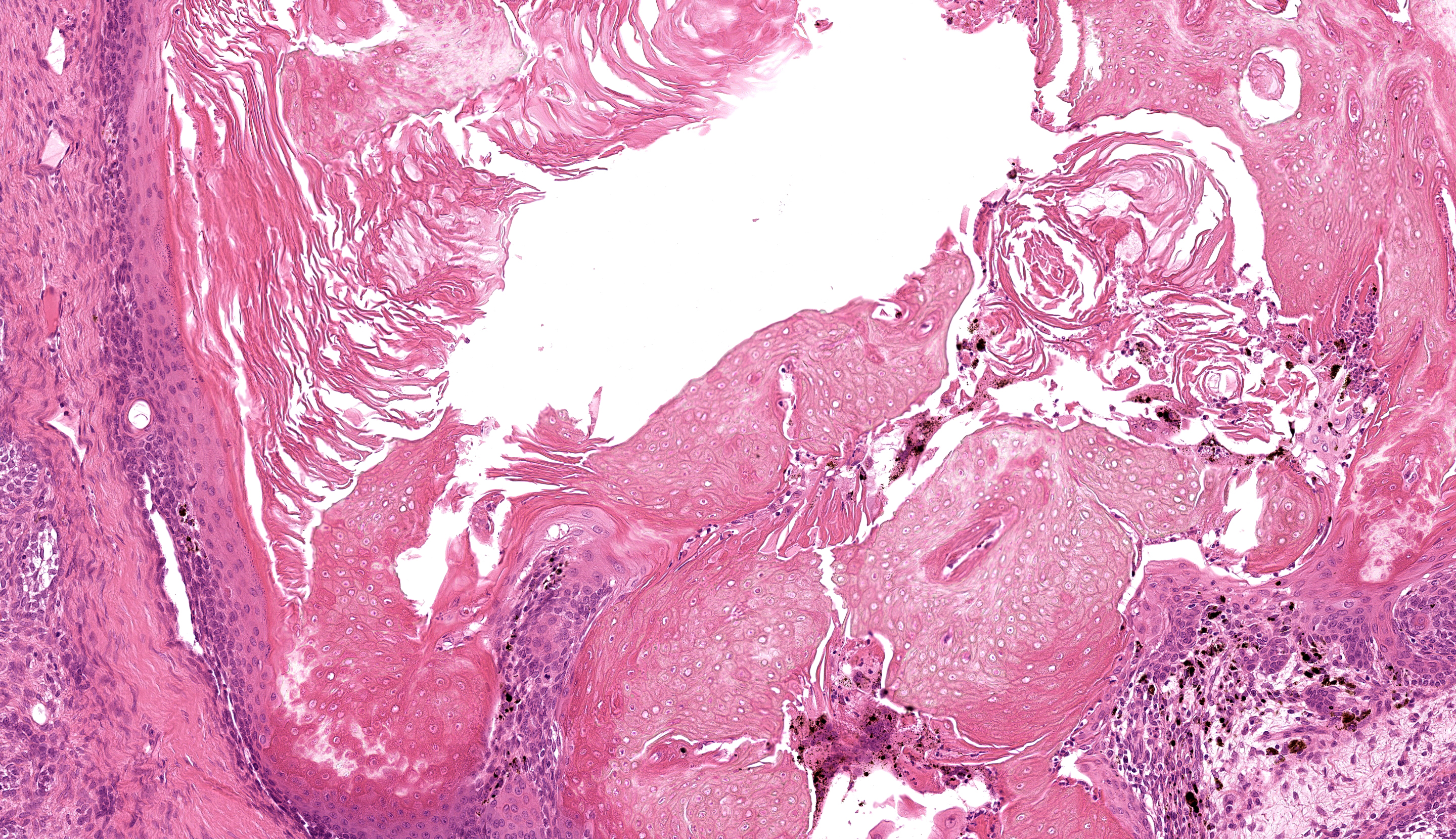
Buccal skin: The lesion comprises a relatively well circumscribed non-encapsulated nodular neoplasm expanding within haired skin which forms the lateral margins. The neoplasm is composed of interconnecting islands and cords of odontogenic epithelium. Odontogenic epithelium is characterized by peripheral palisading of epithelial cells which have apical nuclei and basal cytoplasmic clearing and central cells which are separated by prominent stellate bridges (Fig. 3). Intracytoplasmic melanin granules are a variable feature within this cellular population, multifocally throughout the lesion. Cellular pleomorphism is moderate. Mitoses range from 0-1 per HPF (per 40x objective), with moderate anisocytosis and anisokaryosis. Odontogenic epithelia frequently form concentric nodules (somewhat reminiscent of Pacinian corpuscles, Fig. 3 arrow) and there are areas of slender anastomosing ribbons. Multifocally, there are cystic structures partially or predominantly lined by palisading odontogenic epithelium which contain dyskeratotic hyperkeratotic debris (Fig. 4). Stratified squamous epithelium with para- and orthokeratotic hyperkeratosis lines further cystic structures, forming an extensive central area of invagination within the neoplasm with coalescent columns of squamous epithelial cells and cystic accumulations of orthokeratotic and parakeratotic debris (Fig. 5). Some of these cystic areas have substantial amounts of heterophilic inflammation with epithelial erosion and ulceration and inflammation extending into the subjacent neoplastic populations. Fragments of mucinous salivary gland are present in the deeper connective tissues (consistent with minor salivary gland/buccal origin). The basal margin of resection comprises compressed striated muscle fibers, including atrophic and regenerative fibers, adipocytes, and varying amounts of fibrosis.
Contributor’s Morphologic Diagnosis:
Keratoameloblastoma (keratinizing acanthomatous epulis), with focal squamous differentiation, buccal skin.
Contributor’s Comment: Histological features are consistent with ameloblastoma, a rare neoplastic entity in rabbits, which has only been described in recent years (since 2009). In total only twelve cases have been described in the literature.1-7 According to one recent study of odontogenic neoplasms the prevalence of ameloblastoma in rabbits is around 0.24%.3 As in other domestic species, these are locally aggressive neoplasms and although they are not considered to carry a metastatic risk, they often invade underlying tissues (including alveolar bone) resulting in loss of teeth. Maxillary and mandibular origin have both been described. However, not all typical cases are apparently associated with the cutaneous or mucosal epithelium on clinical inspection, and subcutaneous origin (external to the buccal musculature) has also been described. This may be pertinent to buccal/labial location in this case. Although evidence suggests that these odontogenic-like tumors of the rabbit cheek may be derived from ectopic rests of transformed tooth germ, the histogenesis of these lesions remains unresolved.2
If ameloblastomas are therapeutically irradiated, other malignancies including squamous cell carcinoma, fibrosarcoma and osteosarcoma have been reported to occur, in some cases several months to years following treatment, in other domestic species.8 Keratoameloblastoma is an uncommon histologic subtype (n=2) amongst the twelve ameloblastoma-like lesions recorded in rabbits to date. This subtype is characterized by keratin formation by ameloblastic epithelium and has also been identified as a rare entity in the human literature.9 Squamous differentiation (differentiation of neoplastic epithelium towards a keratinizing squamous epithelial phenotype, rather than keratinization arising from ameloblastic cells) has also been recorded in ameloblastoma in rabbit. Whether this has any prognostic significance has not yet been evaluated.
Contributing Institution:
International Zoo Veterinary Group Pathology
Station House, Parkwood Street
Keighley
West Yorkshire
United Kingdom
BD21 4NQ
https://www.izvg.co.uk/pathology/pabout.htm
JPC Morphologic Diagnosis: Haired skin: Ameloblastoma.
JPC Comment: This third case presented a bit of a conundrum for the group. While the neoplasm description is straightforward, placing an ameloblastoma in the cheek and/or lip without an apparent source of dental germinal epithelium led us to further explore the idea of an ectopic ameloblastoma (see reference #2 below) or back off this interpretation and use the qualifier of “ameloblastoma-like” to allow for the possibility of another cell of origin. Other similar neoplasms that could mimic ameloblastoma due to overlapping features include trichoblastoma (to include “adamantanoid” trichoblastoma) and basal cell adenoma.2 The presence of pigment within neoplastic cells is also not typical of ameloblastoma but is a common finding in follicular tumors which gives some credence to the latter theory of this case.
Dr. Murphy reviewed the Vickers-Gorlin criteria10 for diagnosis of ameloblastoma which include hyperchromatic basal cell nuclei, basilar cell palisading and antibasilar nuclear location, and cytoplasmic vacuolation. We also note the prominent, thickened rim (subepithelial band) of hyalinized collagen that blends with the basement membrane (colloquially the Vickers-Gorlin change or Vickers-Gorlin effect2) though whether this is a consistent or useful feature of rabbit ameloblastomas was not determined by our discussion.
We also discussed the architectural contrast between the deeper portion of this neoplasm necrotic, ulcerated surface and the multifocal keratinization present at the surface. The contributor lays the case nicely for considering keratoameloblastoma. The most recent WHO classification of benign odontogenic tumors makes no mention of this entity however,9 which reflects the need to learn more. One aspect of the slide that conforms to keratoameloblastoma that might be overlooked is the abundant dystrophic mineralization of keratin – this is also noted radiographically in more advanced/pronounced human cases.9
References:
- Rätsep E, Ludwig L, Dobromylskyj M. Orofacial masses in domestic rabbits: a retrospective review of 120 cases from 2 institutions, 2000-2023. J Vet Diagn Invest. 2024 Feb 22:10406387241234326.
- Murphy BG, Swan E, Affolter VK, et al. Odontogenic-like neoplasms of the rabbit cheek: pathological features and comparison to cutaneous trichoblastoma and jaw-associated ameloblastoma. Vet Pathol. 2023 Mar;60(2):178-184.
- Baum B. Not just uterine adenocarcinoma-neoplastic and non-neoplastic masses in domestic pet rabbits (Oryctolagus cuniculus): a review. Vet Pathol. 2021 Sep;58(5):890-900.
- Miwa Y, Nakata M, Takimoto H, et al. Spontaneous oral tumours in 18 rabbits (2005-2015). J Small Anim Pract. 2021 Feb;62(2):156-160.
- Völker I, Kammeyer P, Hinzmann B, Lüerssen D, et al. Peripheres keratinisierendes Ameloblastom bei einem Zwergkaninchen (Oryctolagus cuniculus f. dom.) [Peripheral keratinizing ameloblastoma in a dwarf rabbit (Oryctolagus cuniculus f. dom.)]. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2014;42(5):331-5.
- Loukopoulos P, Komnenou A, Papadimitriou S, et al. Follicular ameloblastoma in a dwarf rabbit (Oryctolagus cuniculus). Proceedings of the European Society of Veterinary Clinical Pathology (ESVCP) and European College of Veterinary Clinical Pathology (ECVCP) 11th Annual Congress. October 7-9, 2009. Thessaloniki, Greece, p. 167
- Madarame H, Enaga S. Peripheral acanthomatous ameloblastoma in a rabbit with review of previous submissions of the Armed Forces Institute of Pathology Wednesday Slide Conference. J Vet Med Sci. 2009 Jul;71(7):987-9.
- Mayer MN, Anthony JM. Radiation therapy for oral tumors: canine acanthomatous ameloblastoma. Can Vet J. 2007 Jan;48(1):99-101.
- Robinson L, Smit C, Fonseca FP, et al. Keratoameloblastoma: A report of seven new cases and review of literature. Head Neck Pathol. 2022 Dec;16(4):1103-1113.
- Vickers RA, Gorlin RJ. Ameloblastoma: Delineation of early histopathologic features of neoplasia. Cancer. 1970 Sep;26(3):699-710.