Signalment:
Gross Description:
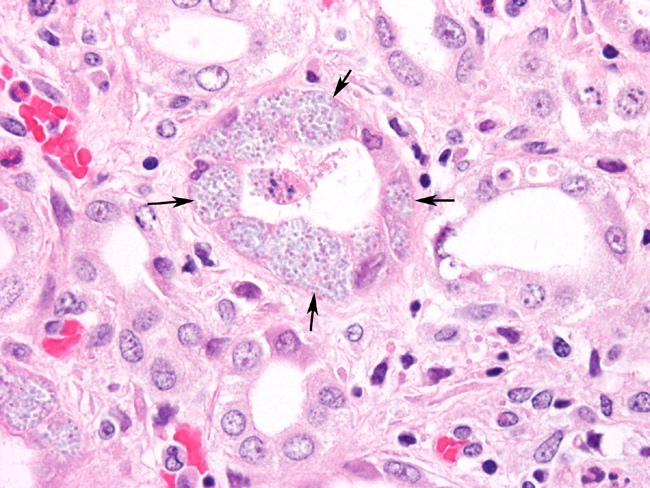
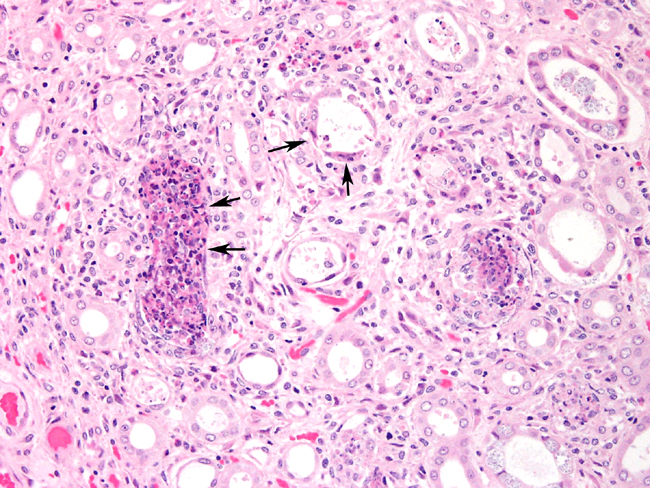
Histopathologic Description:
Morphologic Diagnosis:
Kidney: Pyelitis, lymphoplasmacytic, perivascular mild, with mild transitional epithelial hyperplasia
Lab Results:
Condition:
Contributor Comment:
E. cuniculi was first described as the etiologic agent of infectious motor paralysis in 1922 and was once thought to be a confounding disease affecting mainly laboratory rabbits.Â
E. cuniculi is a member of the phylum Microspora that includes single-celled, spore- forming, obligate intracellular parasites with a direct life cycle. E. cuniculi is the most extensively studied microsporidium that can infect a large variety of mammals.8
Microsporidia are traditionally considered protozoal organisms although novel genetic and molecular evidence suggest closer phylogenetic relationship to fungi. These findings include the presence of a particular mitochondrial heat shock protein that is more closely related to that of fungi as well as the composition of ?- and ?-tubulin resemble more of fungal tubulins. The organism contains chitin and trehalose that are also typical fungal components (reviewed in 9). E. cuniculi has three strains: strain I was found in rabbits and humans, strain II in rodents and blue foxes, and strain III in dogs and humans. Identification of microsporidia species in humans and animals suggest possible zoonotic transmission. In humans, mostly immunocompromised (such as HIV positive patients with AIDS) individuals are infected. Though strain III of E. cuniculi is found in humans and dogs, no direct evidence suggests that dogs can transmit the disease to humans.1
The organism contains a coiled polar filament in the mature spore stage. The sporoplasm following extrusion from the spore coat becomes capable of invading a susceptible host cell. Upon entry multiplication occurs in association with a cytoplasmic vacuole. Sporoblasts develop into mature spores and the ruptured host cell releases the organisms into the surroundings that can then infect other cells.7 The organism does not contain mitochondria and peroxisomes. Intervening DNA sequences (introns) are rare in microsporidial DNA suggesting that the parasite is highly adapted to survive in their hosts.9
Infection in mammals most often occurs by ingestion or inhalation of contaminated urine or feces shed by infected hosts. Also, infection by transplacental transmission and traumatic inoculation has also been described.1 In the large domestic rabbits the infection is usually subclinical and renal lesions are frequently found incidentally. Occasional nervous signs with mortality can occur in young, heavily infected New Zealand rabbits. Dwarf rabbits are especially susceptible. Infection in mice, guinea pigs, nonhuman primates, carnivores and other mammals have also been reviewed.9 In neonatal dogs and blue foxes severe symptoms may develop.3 A serological study in the UK among pet rabbits tested as either companion of infected rabbits or part of a health screen have shown 14 of 38 asymptomatic rabbits to be seropositive. 87 rabbits showing neurological, renal or ocular signs suggestive of encehalitozoonosis were also tested.2
For rabbits, the usual source of infection is spores shed in the urine from actively infected rabbits but they are also readily infected experimentally by oral or respiratory routes. The spores pass to the systemic circulation via infected mononuclear cells. Target organs initially are those with high blood flow such as lung, liver and kidney. At 1 month after oral inoculation moderate to marked lesions can be demonstrated primarily in lung, liver, kidney, and not in the CNS. At 3 months postinoculation, moderate to severe lesions are present in the kidney and minimal changes in heart, lung, liver, and also in brain. Serum titers can be detected at 3-4 weeks and become high at 6-9 weeks. Spores are shed at 1 month in the urine up to 2 months. Shedding terminates at 3 months.7
The parasite is able to infect a large variety of cell types such as neurons, epithelial cells of ependyma and choroid plexus, renal tubular epithelium, endothelium and macrophages.3 Lesions in kidney occur as focal, irregular, depressed, 1-100 mm diameter areas. In severe cases lesions may extend into the underlying cortex. Granulomatous lesions are evident in the interstitium of the lung, kidney and liver at 1 month postinoculation. In the lung, focal to diffuse interstitial pneumonitis with mononuclear cell infiltration may occur. Hepatic lesions are characterized by focal granulomatous inflammatory response with periportal lymphocytic infiltration. Focal lymphocytic infiltrates may also occur in the myocardium. Early lesions in the kidney display focal to segmental granulomatous interstitial nephritis with degenerated epithelial cells and mononuclear cell infiltration. Lesions minimally involve glomeruli. Spores may be present in epithelial cells, macrophages, inflammatory foci, or free within collecting tubules. At 1-2 months postinoculation organisms are already present in kidneys. At a later stage, in renal lesions interstitial fibrosis, collapse of parenchyma, mononuclear cell infiltrations are typical. The organism is eliminated from the kidneys by this time.
In CNS, lesions do not occur until after the 1 month of exposure. Changes are focal nonsuppurative granulomatous meningoencephalomyelitis with astrogliosis and perivascular lymphocytic infiltration. Astroglial cells and granulomatous inflammatory foci contain organisms. Lesions can be detected in the absence of organisms. E. cuniculi infection has been also associated with cataractous changes. Organisms can be identified within the affected lens stroma. These cases are likely caused by intrauterine infections.7 In the nervous system, the lesions are wide spread nonsuppurative meningoencephalomyelitis and the severity varies unpredictably in the different parts suggesting random localization of the organism and irregular distribution of inflammatory vascular changes. Small vessels are surrounded by focal gliosis and microscopic granulomas. Mononuclear cells form cuffs around larger vessels that show segmental fibrinoid change involving the adventitia and perivascular space and eventually appear similar to epithelial cells. Astrocytosis occurs in the surrounding parenchyma. The vascular lesions in the meninges, in the acute infection resemble polyarteritis nodosa and in the chronic disease become dominated by sclerotic changes with persisting perivascular cuffing and granulomatous reactions. Surviving puppies develop progressive renal disease.4
Staining properties of organism and nature of inflammatory response can differentiate it from other infections such as Toxoplasma gondii. Gram positive stains mark organisms as 1.5-2.5 mm in size. Carbon fuchsin stains them purple. In addition, serology tests are available such as modified India ink immunoreaction test as well as indirect immunofuorescent microscopy and a dot ELISA test. An intradermal skin test has been used also to detect infected rabbits.7 Electron microscopy can reveal the organisms in parasitophorous vacuoles as well as the distinctive polar filaments.3
JPC Diagnosis:
Conference Comment:
References:
2. Harcourt-Brown FM, Holloway HK: Encephalitozoon cuniculi in pet rabbits. Vet Rec 152:427-431, 2003
3. Jones TC, Hunt RD, King NW: Veterinary Pathology, 6th Ed., pp. 575 - 578. Lippincott Williams & Wilkins, Baltimore, Maryland, 1997
4. Maxie MG, Youssef S: Nervous system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1, pp. 433-435. Elsevier Limited, St. Louis, MO, 2007
5. Maxie MG, Newman SJ: Urinary system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, p. 479. Elsevier Limited, St. Louis, MO, 2007
6. Newman SJ, Confer AW, Panciera RJ: Urinary system. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 662-664. Elsevier, St. Louis, MO, 2007
7. Percy DH, Barthold SW: Rabbit. In: Pathology of Laboratory Rodents and Rabbits, 3rd ed., pp. 290-294. Blackwell Publishing, Ames, IA, 2007
8. Szabo JR, Shadduck JA: Experiemental encephalitozoonosis in neonatal dogs. Vet Pathol 24:99-108, 1987
9. Wasson K, Peper RL: Mammalian microsporidiosis. Vet Pathol 37: 13-28, 2000
10. Wilcock BP: Eye, eyelids, conjunctiva and orbit. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 1396-1397. Elsevier, St. Louis, MO, 2007
11. Zeman DH, Baskin GB: Encephalitozoonosis in squirrel monkeys (Saimiri sciureus). Vet Pathol 22:24-31, 1985