Signalment:
Gross Description:
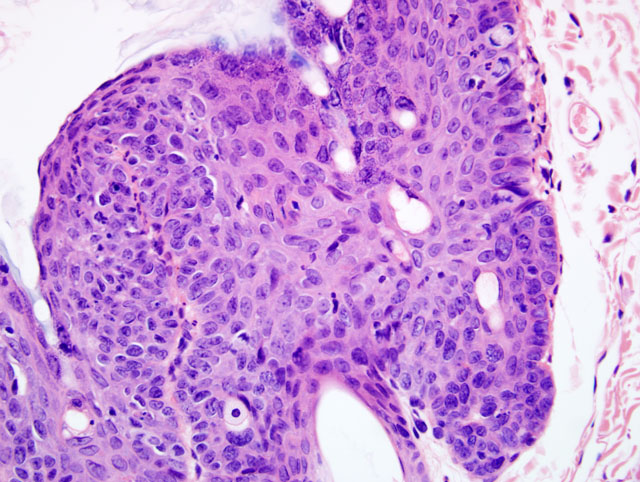
Histopathologic Description:
Morphologic Diagnosis:
Haired Skin: 1. Moderate, multifocal to coalescing, chronic, irregular, follicular and epidermal hyperplasia, hyperkeratosis and dysplasia with occasional koilocytosis
2. Mild, multifocal, subacute, neutrophilic and lymphoplasmacytic superficial and exudative dermatitis
Lab Results:
Blood smear: Rare extracellular Hepatozoon sp. gamonts were observed.
Microbiology: Negative for fungal growth
PCR: Total DNA extracted from the papillomas was positive using FAP59/64 degenerate primers for amplifying sequences within the L1 ORF of papillomaviruses.(1) In situ hybridizations: All DNA in situ hybridization (ISH) probes designed to anneal with BPCV1 DNA sequences failed to stain papillomas from the southern brown bandicoot.(2) All DNA ISH probes designed to anneal with BPCV2 DNA sequences (within the L1, L2 and small t antigen ORFs as well as a BPCV2 genomic DNA probe) stained the nuclei of many keratinocytes within the affected epidermis and root sheath.(3)
Condition:
Contributor Comment:
Multiply primed rolling circle amplification (4) successfully amplified a circular double-stranded DNA genome, which upon restriction enzyme digestion (EcoRI, BamHI, SalI, BglII, HindIII, KpnI) was ~7.3 kilobase pairs (kb). PCR results indicated the presence of papillomavirus-like L1 and L2 ORFs and polyomavirus-like T antigen ORFs1,3,5. DNA sequencing of the amplicons generated through PCR confirmed these findings.(2) The PCR and restriction enzyme digestion results indicated that the current isolate was similar but not identical to bandicoot papillomatosis carcinomatosis virus type 1 (BPCV1).(7,10) This was later confirmed by complete genomic sequencing of the current virus isolate (GenBank# EU277647), which was consequently named bandicoot papillomatosis carcinomatosis virus type 2 (BPCV2).(2)
In situ hybridization results demonstrated that both the papillomavirus-like and polyomavirus-like parts of the BPCV2 genome could be found within the nuclei of keratinocytes of cutaneous papillomatous lesions of the affected southern brown bandicoot.(7)
Bandicoot papillomatosis carcinomatosis virus type 1 was recently discovered in western barred bandicoots (Perameles bougainville) in association with papillomatous and carcinomatous epithelial lesions grossly similar to the lesions evident on the southern brown bandicoot.(1,2,10,11) The BPCVs have certain genomic characteristics typical of Papillomaviridae and other genomic features classically associated with Polyomaviridae5. Their ~7.3 kb double-stranded, circular DNA genomes are similar in size to known papillomaviruses, and they encode structural proteins similar to the L1 and L2 capsid proteins of established papillomavirus types.(2,10) The transforming protein-encoding ORFs, most similar to large T antigen and small t antigen, occur on the opposite DNA strand to the structural protein-encoding ORFs: features characteristic of viruses classified within the Polyomaviridae.(2,10)
Mitochondrial DNA evidence suggests the two extant genera, within the family Peramelidae, Isoodon and Perameles, diverged from a common bandicoot ancestor approximately 10 million years ago.(2,5) The divergence of the host genera from a common ancestor appears to approximately coincide with the divergence of the BPCVs affecting them.(2) This observation is supportive of the concept of virus-host co-speciation in which both modern day hosts and viruses arose from common ancestors.(6,8)
As it stands, the current virus taxonomic paradigm does not comfortably accommodate the BPCVs whose genomic features are intermediate between Papillomaviridae and Polyomaviridae.(2,10) It is clear the BPCVs are demonstrably and distinctly different to both polyomaviruses and papillomaviruses and as such, their taxonomic position is presently undefined.
The presence of Hepatozoon sp. gamonts in blood smears was considered an incidental finding. The prevalence of Hepatozoon sp. infection in I. obesulus from Perth, Western Australia was 48% by examination of stained blood smears and 58% by PCR of DNA extracted from blood in a recent survey.(9)
JPC Diagnosis:
Conference Comment:
Papilloma viruses, of the Papoviridae family, are double stranded DNA viruses that form paracrystalline arrays. Cutaneous papillomas of viral origin are quite common in domestic animals. Papillomas can either be viral induced or an idiopathic proliferation of the epidermis. With the exception of bovine papilloma viruses, papilloma viruses are normally site and species specific. Bovine papilloma viruses have been linked to feline cutaneous fibropapillomas and equine sarcoids. There are two general categories of viral induced papillomas, the squamous papilloma and the fibropapilloma.(4)
Squamous papillomas are filiform, exophytic, wart-like masses with marked epidermal hyperplasia and either orthokeratotic or parakeratotic hyperkeratosis with support provided by a thin dermal stalk. The stratum spinosum is markedly acanthotic, and the cytoplasm of virally infected cells may exhibit ballooning degeneration with eccentrically placed nuclei. These cells are known as koilocytes and are a helpful histologic feature. There is also hypergranularity of the stratum granulosum characterized by large, abnormally shaped eosinophilic granules within the cytoplasm. Eosinophilic, intracytoplasmic inclusions represent aggregates of keratin in dying keratinocytes and should not be confused with pox inclusions. Small, rare, basophilic intranuclear inclusions can also occur.(4)
Fibropapillomas, represented by equine sarcoids and feline fibropapillomas, are nodular lesions covered by a hyperkeratotic and hyperplastic epidermis with rete ridge formation. The predominant feature of these lesions is the marked expansion of the dermis by proliferating fibroblasts arranged in haphazard whorls.(4)
References:
2. Bennett MD, Woolford L, Stevens H, Van Ranst M, Oldfield T, Slaven M, OHara A J, Warren KS, Nicholls PK: Genomic characterization of a novel virus found in papillomatous lesions from a southern brown bandicoot (Isoodon obesulus) in Western Australia. Virology 376:173-182, 2008
3. Forslund O, Antonsson A, Nordin P, Stenquist B, Hansson BG: A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J Gen Virol 80:2437-2443, 1999
4. Ginn PE, Mansell JEKL, Rakich PM: Skin and appendages. In: Jubb, Kennedy and Palmer's Pathology of Domestic Animals, ed. Maxie MG, 5th ed., pp. 748-751. Elsevier Saunders, Philadelphia, PA, 2007
5. Nilsson MA, Arnason U, Spencer PBS, Janke A: Marsupial relationships and a timeline for marsupial radiation in South Gondwana. Gene 340:189-196, 2004
6. Rector A, Lemey P, Tachezy R, Mostmans S, Ghim S-J, Van Doorslaer K, Roelke M, Bush M, Montali RJ, Joslin J, Burk RD, Jenson AB, Sundberg JP, Shapiro B, Van Ranst M: Ancient papillomavirus-host co-speciation in Felidae. Genome Biol 8:R57, 2007
7. Rector A, Tachezy R, Van Ranst M: A sequence-independent strategy for detection and cloning of circular DNA virus genomes by using multiply primed rolling-circle amplification. J Virol 78:4993-4998, 2004
8. Shadan FF, Villarreal LP: Coevolution of persistently infecting small DNA viruses and their hosts linked to host-interactive regulatory domains. Proc Natl Acad Sci USA 90:4117-4121, 1993
9. Wicks RM, Spencer PBS, Moolhuijzen P, Clark P: Morphological and molecular characteristics of a species of Hepatozoon Miller, 1908 (Apicomplexa: Adeleorina) from the blood of Isoodon obesulus (Marsupialia: Peramelidae) in Western Australia. System Parasitol 65:19-25, 2006
10. Woolford L, Rector A, Van Ranst M, Ducki A, Bennett MD, Nicholls PK, Warren KS, Swan RA, Wilcox GE, OHara AJ: A novel virus detected in papillomas and carcinomas of the endangered western barred bandicoot (Perameles bougainville) exhibits genomic features of both the Papillomaviridae and Polyomaviridae. J Virol 81:13280-13290, 2007
11. Woolford L, OHara AJ, Bennett MD, Slaven M, Swan R, Friend JA, Ducki A, Sims C, Hill S, Nicholls PK, Warren KS: Cutaneous papillomatosis and carcinomatosis in the western barred bandicoot (Perameles bougainville). Vet Pathol 45:95-103, 2008