Signalment:
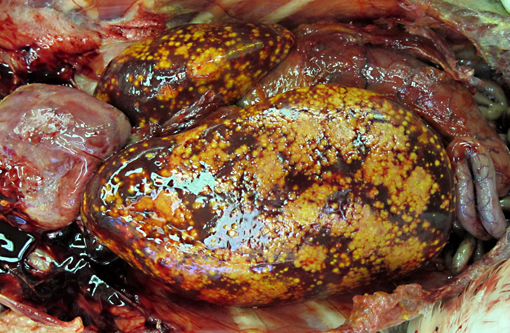
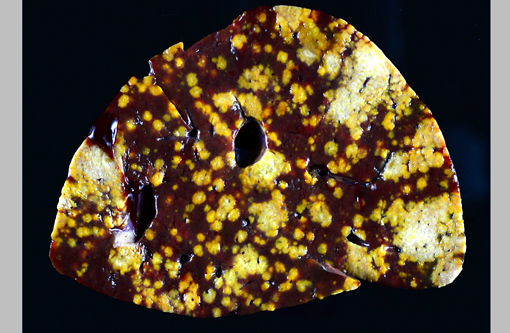
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
1. Liver: severe, acute to subacute, multifocal to coalescing, necrotizing hepatitis with intralesional protozoal trophozoites (Tetratrichomonas gallinarum).Â
2. Liver: moderate extramedullary hematopoiesis.
Lab Results:
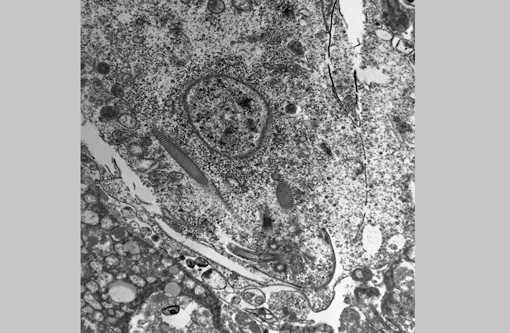
Electron microscopy (formalin-fixed liver): Flagellated protozoal organisms consistent with a trichomonad were seen. On transmission EM within areas of necrosis there were numerous 10.3 X 6.3 μm single-celled microorganisms with 4 anterior flagella, one recurrent flagellum, basal bodies, pelta, axostyle, costa, hydrogenosomes, and glycogen granules. With scanning EM, round to oval spherules were mixed with cell debris. Occasionally these organisms had four anterior flagella and a single recurrent flagellum, but most lacked flagella and an undulating membrane.Â
Condition:
Contributor Comment:
The appearance of the hepatic lesions on gross and histopathologic examination was similar to those of histomoniasis caused by the trichomonad Histomonas meleagridis. The pleomorphic size and shape of the organisms was comparable to that of H. meleagridis in its amoeboid phase, the form that occurs in tissues.(6) Therefore, a similar change in morphology between organisms in the intestinal lumen or culture and those in tissue might occur with T. gallinarum. The presence of basal bodies and the variable presence of flagella of the organisms in the liver lesions were seen with electron microscopy and consistent with the PCR results. The presence of apparently multinucleated forms on routine histopathology in this case was unusual and was not appreciated on EM. Replication by binary fission and dense crowding of the organisms could possibly account for this appearance.Â
To our knowledge, there are no reports of Tetratrichomonas sp. infections in pelicans, and the reason for disease in this bird is unknown. The liver and spleen were most severely affected with necrotizing lesions effacing large areas of the parenchyma, but smaller foci of necrosis with intralesional organisms were also occasionally seen in the lung, heart, skeletal muscle, bone marrow, proventriculus and intestine, consistent with hematogenous dissemination of the infection. The likely origin of these trichomonads was the intestinal tract, although no areas of colonization of the lumen or crypts were seen. However, in addition to the variety of endoparasites noted grossly, myriad small trematodes invaded and multifocally disrupted the small intestinal, colonic and cecal mucosa. Secondary bacterial infections were also present in the ceca, and it is possible that this damage to the intestinal mucosa allowed invasion and dissemination of the flagellates. Poor immune function could also have been a contributing factor, as this pelican was young, heavily parasitized and in poor nutritional condition. In a previous report of this infection in ducks, all affected ducks were juveniles or subadults.(9)
Finally, as previously noted, isolates of T. gallinarum have a high degree of molecular polymorphism which could indicate the presence of multiple species or subspecies within this group, many of which could be host-adapted.(3) Infection of a non-galliform or anseriform bird (such as a pelican) or cross-infections between galliforms and anseriforms, might be more likely to result in disease. A previous report of encephalitis due to T. gallinarum was in a mockingbird, a passerine bird, and in the previous outbreak in ducks, a turkey was a possible source of the parasite.(8,9) The pelican in this report could have been exposed to a variety of native and exotic anseriform and possibly galliform birds at our zoological institution, none of which to date have been found to have a disseminated form of this infection.
JPC Diagnosis:
Conference Comment:
As the contributor notes, the pathogenicity of Tetratrichomonas gallinarum is unclear, as it is often identified in the cecum and colon of domestic species without causing disease.(2) However, recently a case of fatal hepatic tetratrichomoniasis was reported in a captive-raised juvenile Waldrapp ibis (Geronticus eremita).(5) Similar to the pelican in this case, the Waldrapp ibis had necrotizing hepatitis associated with numerous protozoa. The protozoa showed positive immunoreactivity to antibodies against Tritrichomonas foetus. RNA sequence analysis (of the first ribosomal internal transcribed spacer region (ITS1), 5.8S ribosomal RNA, and ITS2 regions) revealed 96-97% genetic similarity to members of the Tetratrichomonas gallinarum complex. Cross-species transmission is suspected as the source of infection in this case as well. Further studies are needed to fully elucidate the pathogenicity of members of the T. gallinarium complex and their role in disease in wild and captive birds.Â
References:
2. Bowman DD. Protozoans. In: Georgis Parasitology for Veterinarians. 9th ed. St. Louis, MO: Saunders Elsevier; 2009:87-89.Â
3. Cepicka I, Kutisova K, Tachezy J, Kulda J, Flegr J. Cryptic species within the Tetratrichomonas gallinarum species complex revealed by molecular polymorphism. Vet Parasitol. 2005;128:11-21.
4. Friedhoff KT, Kuhnigk C, Muller I. Experimental infection in chickens with Chilomastix gallinarum, Tetratrichomonas gallinarum, and Tritrichomonas eberthi. Parasitol Res. 1991;77:329-334.
5. Laing ST, Weber ES 3rd, Yabsley MJ, Shock BC, Grosset C, Petritz OA, et al. Fatal hepatic tetratrichomoniasis in a juvenile Waldrapp ibis (Geronticus eremita) [abstract]. J Vet Diagn Invest.; 2013: Feb 12. [Epub ahead of print]. Accessed online at http://www.ncbi.nlm.nih.gov/pubmed/23404476 on 17 February 2013.Â
6. McDougald LR. Other protozoan diseases of the intestinal tract. In: Calnek BW, ed. Diseases of Poultry. 10th ed. Ames, IA: Iowa State University Press; 1997:890-899.
7. Norton RA. Pathogenicity of a strain of Trichomonas gallinarum in turkeys and its possible interaction with cecal coccidia. Avian Dis. 1997;41:670-675.
8. Patton CS, Patton S. Tetratrichomonas gallinarum encephalitis in a mockingbird (Mimus polyglottos). J Vet Diagn Invest. 1996;8:133-137.
9. Richter B, Schulze C, Kammerling J, Mostegl M, Weissenbock H. First report of typhlitis/typhlohepatitis caused by Tetratrichomonas gallinarum in three duck species. Avian Pathol. 2010;39(6):499-503.