Signalment:
Gross Description:
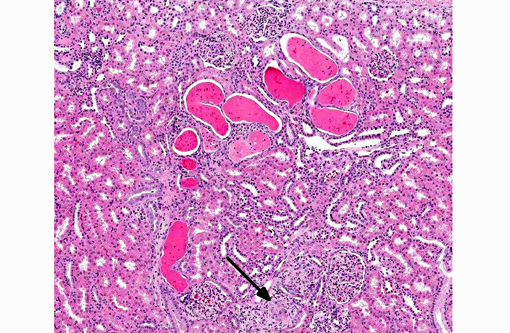
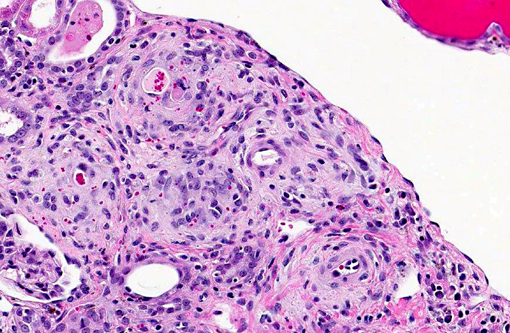
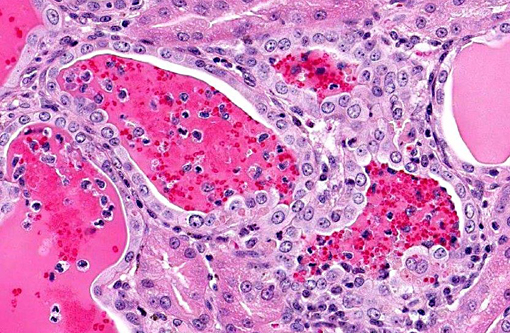
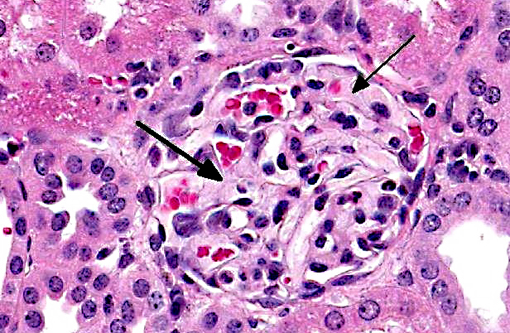
Histopathologic Description:
Morphologic Diagnosis:
Kidney:
- Marked multifocal arteriopathy
- Moderate multifocal subacute renal cortical tubular degeneration and regeneration with tubular dilation and casts
- Mild multifocal membranoproliferative glomerulopathy
- Minimal multifocal chronic interstitial nephritis
Lab Results:
Condition:
Contributor Comment:
A recent review paper on calcineurin inhibitor nephrotoxicity in humans describes the chronic morphologic features as nodular hyaline material deposition in the tunica media of afferent arterioles, tubular atrophy, interstitial fibrosis, and glomerular sclerosis, with the hallmark feature being the arteriolar lesion.(5) It has been speculated that the non-vascular renal changes, which are said to have a "stripe-like" distribution, are due, at least in part, to ischemia resulting from the renal arteriolar changes.(4,5) Similarly, the arteriolar changes are also thought to be responsible for the associated hemolytic anemia because they result in mechanical damage and eventual intravascular hemolysis of red blood cells.(2)
This case was complicated by the fact that this animal also had a mononuclear meningitis, suggestive of a viral etiology (although no viral inclusion bodies were noted microscopically) secondary to cyclosporine-induced immunosuppression. Primary differentials for meningoencephalitis include simian virus 40 (SV-40), which can also cause interstitial nephritis,6 and herpesviral infection, specifically cytomegalovirus (CMV).(1)
Immunohistochemistry on affected areas of brain was attempted and was negative for SV-40, but CMV antibodies were not reactive against macaque CMV.
JPC Diagnosis:
1. Kidney, arterioles: Arteritis, proliferative, diffuse, moderate.Â
2. Kidney, tubules: Degeneration and necrosis, multifocal, mild with tubular ectasia and protein casts.Â
3. Kidney: Glomerulonephritis, membranous, diffuse, moderate.
Conference Comment:
Acute CNI nephrotoxicity results from vasoconstriction of the afferent arteriole due to increased levels of vasoconstrictors (such as endothelin, thromboxane and activation of the renin-angiotensin system) and decreased quantities of vasodilators (such as prostaglandin-E2, prostacyclin, and nitric oxide). This dose-dependent and reversible arteriolar vasoconstriction results in reduced renal blood flow, reduced glomerular filtration, increased renal vascular resistance and tubular dysfunction.(8) Chronic CNI nephrotoxicity is also associated with impairment of renal function and is likely mediated by several different growth factors and cytokines, including transforming growth factor beta (TGFβ), platelet derived growth factor (PDGF), fibroblast growth factor (FGF) and TNF-α. CNIs have been shown to produce various side effects in addition to nephrotoxicity, including diabetes, neurological dysfunction, hemolytic anemia, hemolytic uremic syndrome, hypertension and hypercholesterolemia.(2,9,10)
Although there is significant tubular degeneration/regeneration as well as mild glomerulonephritis and glomerulosclerosis, conference participants agree that the most striking lesions in this interesting case are the occlusive arteriolar changes, consisting of disruption of the internal elastic membrane, proliferation, hypertrophy, hyalinosis and fibrosis within the tunica intima/media and significant narrowing of affected lumina. Similar changes are reported in association with chronic allograft nephropathy (characterized by progressive and irreversible deterioration of renal function with interstitial fibrosis, tubular atrophy, arteriolar hyalinosis, and glomerulosclerosis) and systemic hypertension.(8) In addition to the microscopic lesions described above, which are consistent with cyclosporine nephrotoxicity, there is evidence of microangiopathic hemolytic anemia, supported by the finding of regenerative anemia on the CBC and the detection of schistocytes, which are fragmented erythrocytes suggestive of shearing of erythrocyte membranes, on the peripheral blood smear. Microangiopathic hemolytic anemia is another well-described sequela to administration of CNIs,(2,9) presumably secondary to erythrocyte damage from the turbulence that results from vasoconstriction and occlusion of renal arterioles. Although further clinical-pathologic data is not available, hemoglobinemia and hemoglobinuria, as well as hyperbilirubinemia, are also expected findings in cases of microangiopathic anemia.(11)
References:
1. Baskin, GB. Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. Am J Pathol. 1987;129(2):345-352.
2. Danesi R, Del Tacca M. Hematologic toxicity of immunosuppressive treatment. Transplantation Proceedings. 2004;36:703-704.
3. Kindt MV, Kemp R, Allen HL, Jensen RD, Patrick DH. Tacrolimus toxicity in rhesus monkey: model for clinical side effects. Transplantation Proceedings. 1999;31:3393-3396.
4. Myers BD, Newton L. Cyclosporine-induced chronic nephropathy: an obliterative microvascular renal injury. Journal of American Society of Nephrology. 1991;2:S45-S52.
5. Naesens M, Kuypers DRJ, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clinical Journal American Society Nephrology. 2009;4:481-508.
6. Simon MA, Ilyinshii PO, Baskin GB, Knight HY, Pauley DR, Lackner AA. Association of simian virus 40 with a central nervous system lesion different from progressive multifocal leukoencephalopathy in macaques with AIDS. Am J Pathol. 1999;154(2):437-446.
7. Wijkstrom M, Kirchhof N, Graham M, Ingulli E, Colvin RB, Christians U, et al. Cyclosporine toxicity in immunosuppressed streptozocin-diabetic nonhuman primates. Toxicology. 2005;207:117-127.
8. Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol. 2013;37(6):602-612.
9. Fellstrom B. Cyclosporine nephrotoxicity. Transplant Proc. 2004;36(2 Suppl):220S-223S.Â
10. Busauschina A, Schnuelle P, van der Woude FJ. Cyclosporine nephrotoxicity. Transplant Proc. 2004;36(2 Suppl):229S-233S.Â
11. Brockus CW. Erythrocytes. In: Latimer KS, ed. Duncan and Prasses Veterinary Laboratory Medicine Clinical Pathology. 5th ed. Ames, IA: Wiley-Blackwell, 2011:30-35.