Signalment:
On clinical presentation, the dog had pale and icteric mucous membranes. Her femoral pulses were weak and intermittent with marked deficits. Electrocardiography suggested atrial fibrillation. The dog had tachypnea and increased respiratory effort, with harsh lung sounds on thoracic auscultation.
Acute oliguric renal failure was diagnosed based on clinical signs and laboratory data (see below). Despite fluid therapy, diuretics and treatment for hyperkalemia, three days following the commencement of clinical signs the dog developed ventricular fibrillation, which progressed to cardiopulmonary arrest that was refractory to resuscitation including defibrillation.
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
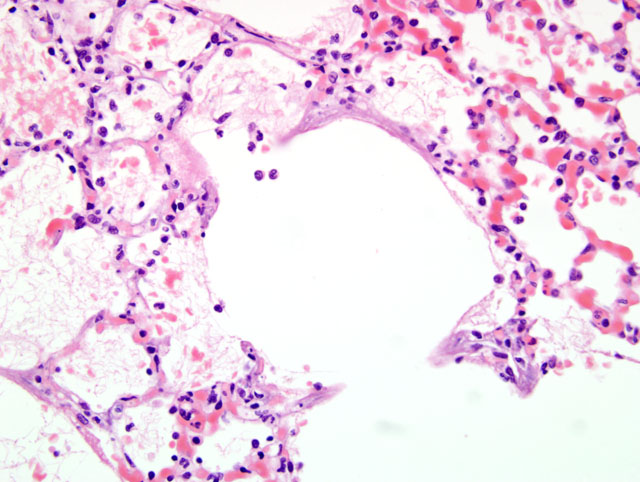
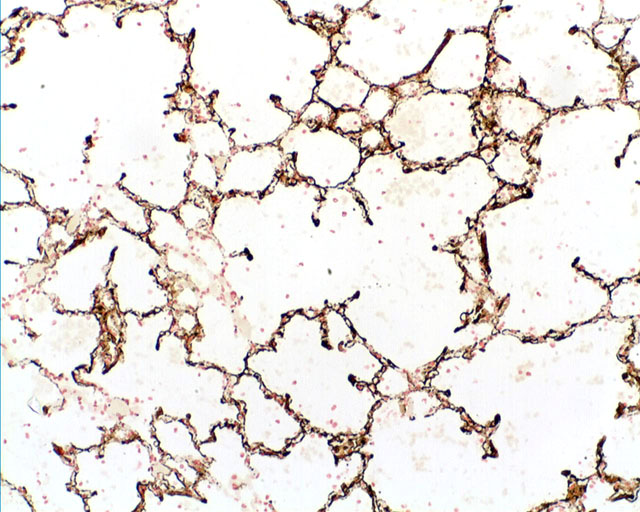
Lungs, alveolar septae; Mineralization with histiocytosis, moderate, diffuse
Lungs, alveoli: Fibrin exudation, moderate with histiocytosis
Lab Results:
Serum biochemistry revealed a moderate panhypoproteinemia 41g/L (reference value: 49-71g/L) due to hypoalbuminemia 21.2g/L (reference value: 28-39g/L) and minimal hypoglobulinemia 19.8g/L (reference value: 21-41 g/L). Further abnormalities included marked hyperkalemia 9.9mmol/L (reference value: 4.1-5.3 mmol/L), marked hypercalcemia 4.12mmol/L (reference value: 2.13-2.7 mmol/L) and marked hyperphosphatemia 6.89mmol/L (reference value: 0.8-2.0 mmol/L). A marked azotemia with elevated blood urea nitrogen (BUN) 64.1mmol/L (reference value: 3-9.1 mmol/l) and elevated creatinine 601μmol/L (reference value 98-163mmol/L) was also present. Other tested parameters included increased amylase 1874U/L (reference value: 176-1245U/L), increased lipase 4470U/L (reference value: 72-1115U/L), hyperbilirubinemia 92.1mmol/L (reference value: 0-2.4 mmol/L) and an increased alkaline phosphatase (ALP) 933 U/L (reference value: 19-285 U/L).
Urinalysis revealed isosthenuria (specific gravity 1.010), pH 7.0 and elevated glucose (3+). Urine culture revealed no bacterial isolates after 48 hours incubation.
Antibody titers to several Leptospira spp., which can be pathogenic to dogs, were tested. All Leptospira serovar pool titers were negative.
Condition:
Contributor Comment:
1) Hyperparathyroidism: Hyperparathyroidism is associated with elevated parathyroid hormone/parathormone (PTH) causing bone resorption and thus hypercalcemia. Primary hyperparathyroidism is characterized by the autonomous secretion of PTH due to parathyroid gland neoplasia.(2,8) Pseudohyperparathyroidism is caused by the secretion of PTH-like proteins by malignant tumors (hypercalcemia of malignancy) e.g. lymphoma and anal sac apocrine gland adenocarcinoma.(2,8) Non-parathyroid neoplasia has been reported as the most frequent cause of hypercalcemia in dogs and cats.(4)
2) Bone destruction: Hypercalcemia can be observed with osteolytic lesions caused by multiple myeloma and tumor metastasis to bone.(5)
3) Vitamin D intoxication: Ingestion of increased amounts of vitamin D analogues, e.g. calcinogenic plants, dietary supplements, rodenticide baits, or other calcinogenic products are other possible causes of hypercalcemia.(5,6)
4) Certain types of granulomatous disease can lead to hypercalcemia, for example blastomycosis in dogs. Activated macrophages have the ability to synthesize the active form of vitamin D3, calcitriol.(5)
5) Renal failure (please see below): Renal failure is associated with phosphate retention (hyperphosphatemia). The increased serum phosphorus (PO4) forms complexes with serum calcium (Ca2+) and metastatic tissue mineralization develops if the product of Ca2+ x PO4 exceeds a certain threshold value.(13)
In the present case, metastatic calcification was observed. In addition to the pulmonary mineralization, mineral deposition was present in the gastric mucosa, renal tubular epithelial cells and renal vessel walls. On macroscopic and microscopic examination of the dog and multiple tissues, the parathyroid glands were unremarkable and no evidence of neoplasia was found. The laboratory data indicated renal failure due to the concurrent presence of hypoalbuminemia, hyperphosphatemia and hyperkalemia and increased BUN and creatinine. The hypoalbuminemia was likely caused by renal loss of protein. The cause of the mild hypoglobulinemia is uncertain. No intestinal lesions were observed at post mortem examination. Hyperphosphatemia, hyperkalemia and increased BUN and creatinine develop following decreased glomerular filtration. The increased levels of amylase and lipase were also likely related to the renal disease, since no lesions suggestive of pancreatitis were observed. Interestingly, the dog also had hypercalcemia. In dogs with renal failure, there are usually hyperphosphatemia and hypocalcemia. In some cases, however, hypercalcemia has been described.(13) The elevated ALP and hyperbilirubinemia suggest decreased hepatic function. The young age of the dog may have contributed to the elevated calcium, phosphorus and ALP levels. Renal failure is classified as pre-renal, renal or post-renal renal failure. The main features of the different types of renal failure are listed below.Â
Pre-renal renal failure is related to a reduced perfusion of the kidneys, which can be caused by low cardiac output, increased renal vasculature resistance, extra-cellular fluid volume depletion or low systemic vascular resistance.(10) Renal renal failure is caused by impaired renal function, which could be related to tubular injury, glomerulonephritis, tubulointerstitial nephritis, acute vascular nephropathy or neoplasia.(10) Post-renal renal failure is caused by urinary tract obstruction, e.g. tubular precipitation, urethral obstruction or bladder obstruction.(10)
In this case, the absence of obstruction in the urinary tract rules out post-renal failure. Because of the history of vomiting and diarrhea, reduced renal perfusion has to be considered as a possible cause of the renal failure. Microscopic examination of the kidneys revealed the presence of mineralization in some tubular epithelial cells and in the walls of occasional blood vessels. Furthermore, mild tubular degeneration and regeneration were observed. The degeneration affected approximately 20-30% of tubules in the cortex and medulla. In addition, the renal interstitium contained a mild multifocal infiltrate of lymphocytes and plasma cells. Thus, pre-renal and renal causes likely contributed to the renal failure. Reportedly, vomiting and diarrhea develop prior to the acute renal failure. Vomiting and diarrhea, however, can also be observed secondary to renal failure.(5,10)
In secondary renal hyperparathyroidism raised levels of ionized plasma phosphate cause hypocalcemia through the precipitation of calcium and phosphorus. The reduction in ionized serum calcium stimulates PTH secretion and the mobilization of calcium from bone stores. This results in bone resorption with the release of phosphorus and calcium. The stimulation of PTH secretion is compounded by the damaged kidneys, which have a reduced capacity to hydroxylate the inactive form of vitamin D 25-hydroxycholecalciferol to the active form of vitamin D, i.e. 1,25-dihydroxycholecalciferol (calcitriol). Thus, the intestinal absorption of calcium is reduced. Calcitriol, which is also suppressed by hyperphosphatemia, normally inhibits PTH secretion. By reducing calcitriol production a physiological control on PTH secretion is removed and eventually parathyroid chief cell hyperplasia occurs, a process termed renal secondary hyperparathyroidism.(12)
PTH enhances calcium release from bone by activating osteoclast resorption through their indirect stimulation. Osteoclasts do not have a receptor for PTH, thus PTH binds to osteoblasts which stimulate osteoblasts to increase their expression of receptor activator of NFκB ligand (RANK ligand). RANK ligand binds to osteoclast precursors containing receptor activator of NFκB (RANK). The binding of RANK ligand to RANK stimulates these precursors to fuse and to form osteoclasts which ultimately enhances the resorption of bone.(3)
The biochemical abnormality characterized by elevated BUN and creatinine is termed azotemia and when this is associated with clinical signs and other biochemical abnormalities it is termed uremia.(10) Non-renal lesions of uremia include pulmonary edema and soft tissue mineralization. In uremia, pulmonary edema most likely occurs via two mechanisms a) mineralization of alveolar septae leading to diffuse alveolar damage and b) uremic toxins causing increased permeability of alveolar capillaries with leakage of protein-rich fluid containing fibrin.(10) Histiocytic cells, including alveolar macrophages have a role in preventing the accumulation of native proteins within alveoli and thus their increased infiltration would be expected in response to proteinaceous edema containing fibrin. In addition, the mineral deposits stimulate a foreign body response causing histiocytic infiltration.(10)
The pathogenesis of soft tissue mineralization in the uremic animal is not fully understood, however, deposition of calcium phosphate mineral favors tissues with an internal alkaline compartment, principally gastric mucosa, kidneys, lungs, systemic arteries and pulmonary veins but other local factors such as tissue glycosaminoglycans may play a role.(7) Renal mineralization compounds the problem by causing further damage and thus a further reduction in glomerular filtration.Â
In the present case, the pulmonary lesions might be caused by uremia. Due to the concurrent presence of hyperphosphatemia and hypercalcemia, however, hypervitaminosis D cannot be ruled out.(5)
Leptospirosis has also been considered as one possible cause of concurrent elevated renal and hepatic parameters in dogs.(1,10) Canine infection is caused by Leptospira (L.) interrogans or L. kirschneri and many pathogenic serovars have been identified with Leptospira canicola, icterohaemorrhagiae, grippotyphosa, pomona and bratislava being the most commonly identified in dogs.(9,14) Following cutaneous or mucosal penetration, leptospires are disseminated via the blood to target tissues such as the kidneys, liver, spleen and central nervous system. The extent of damage to internal organs varies with the virulence of the organism and host susceptibility. In addition, some serovars show a predilection for the liver or kidney. The mechanisms by which leptospires cause disease are not well understood, and leptospiral lipopolysaccharide may play a role in inflammatory and coagulatory abnormalities that occur. Renal damage occurs following colonization and replication of the organisms in renal tubular epithelial cells, whereas histochemical studies have shown that the fundamental hepatic lesion is due to subcellular effects on enzyme systems.(9) In this case the standard serologic test, microscopic agglutination test (MAT), was performed however titers to all tested serovars were negative. It has been reported that in acute or peracute disease antibody responses have not yet developed and as such serological tests are not useful until 7 to 10 days after infection.(1,9)
JPC Diagnosis:
Conference Comment:
In reviewing the clinical chemistry data from this case, the moderator emphasized to attendees that an increase in ALP may occur for a variety of reasons, e.g. cholestasis, steroid induction. Furthermore, there may have been increased activity of the bone isoenzyme of ALP due to the young age of the puppy in the case, or perhaps increased osteoclastic activity due to the suspected renal hyperparathyroidism. Most importantly, ALP is independent and non-predictive of hepatic function. Analysis of pre- and post-prandial bile acid concentration is the only direct method to assess hepatic function; albumin and BUN concentrations (if low) may provide indirect clues to the status of liver function.
The moderator and conference participants also prefer the use of the term azotemia versus renal failure in the otherwise well-crafted contributor section on pre-, renal and post-renal azotemia. Renal failure, defined as an increase in BUN/Creatinine and decreased urine specific gravity, is present in this case.
References:
2 Capen CC: Endocrine glands. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, ed. Maxie GM, 5th ed., pp. 363-375. Elsevier Limited, St Louis, MO, 2007
3 Capen CC: Endocrine glands. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, ed. Maxie GM, 5th ed., pp. 355-356. Elsevier Limited, St Louis, MO, 2007
4 Elliott J, Dobson JM, Dunn JK, Herrtage ME, Jackson KF: Hypercalcaemia in the dog: a study of 40 cases. J Small Anim Pract 32:564-571, 1991
5 Ferguson DC, Hoenig M: Endocrine system. In: Duncan & Prasse's Veterinary Laboratory Medicine Clinical Pathology, eds. Latimer KS, Mahaffey EA and Prasse KW, 4th ed., pp. 270-278. Blackwell, Ames, Iowa, 2003
6 Hilbe M, Sydler T, Fischer L, Naegeli H: Metastatic calcification in a dog attributable to ingestion of a tacalcitol ointment. Vet Pathol 37:490-492, 2000
7 Kumar V, Abbas AK, Fausto N: General Pathology - Cellular adaptations, Cell injury, and Cell death. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK and Fausto N, pp. 41-42. Elsevier Saunders, Philadelphia, PA, 2005
8 La Perle KMD, Capen CC: Endocrine system. In: Pathologic Basis of Veterinary Disease, eds. McGavin DM and Zachary JF, 4th ed., pp. 731-734. Mosby, St Louis, MO, 2007
9 Langston CE, Heuter KJ: Leptospirosis. A re-emerging zoonotic disease. Vet Clin North Am Small Anim Pract 33:791-807, 2003
10 Maxie GM, Newman SJ: Urinary system. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, ed. Maxie GM, 5th ed., pp. 432-436. Elsevier Limited, St Louis, MO, 2007
11 Maxie GM, Robinson WF: Cardiovascular system. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, ed. Maxie GM, 5th ed., pp. 61-62. Elsevier Limited, St Louis, MO, 2007
12 Newman SJ, Confer AW, Panciera RJ: Urinary system. In: Pathologic Basis of Veterinary Disease, eds. McGavin DM and Zachary JF, 4th ed., pp. 641-644. Elsevier Limited, St Louis, MO, 2007
13 Stockham SL, Scott MA: Calcium, phosphorus, magnesium, and their regulatory hormones. In: Fundamentals of Veterinary Clinical Pathology, eds. Stockham SL and Scott MA, 1st ed., pp. 401-417. Blackwell, Ames, Iowa, 2002
14 Stokes JE, Forrester SD: New and unusual causes of acute renal failure in dogs and cats. Vet Clin North Am Small Anim Pract 34:909-922, vi, 2004