Signalment:
>20-month-old male Sprague-Dawley rat (
Rattus norvegicus).The rat had 4ET
telemetry device implantation surgery on 28 October 2014 & was exposed to
test article on 8 December 2014. The rat had two routine battery exchange
surgeries on 24 February 2015 and 22 July 2015. Post-op recovery was
uneventful. In early September 2015, the rat developed fluid-filled distention
on both flanks. Veterinary staff attempted to manually drain several times,
but size remained the same. On 10 Sept 2015, a veterinarian performed a
battery exchange. During the surgery, the surgeon removed fibrous/sac-like
tissues (submitted for biopsy) from both sides while draining a copious amount
of serosanguineous fluids (no purulent fluid noted). The rat was placed on
enrofloxacin (1mg/kg) post-surgery. On 23 September 2015, chlorhexidine scrub
& triple antibiotic ointment treatment was started because the area at the
incision site appeared raw. The rat appeared bright, alert, and responsive
during rounds on 25 Sept 2015, but was found dead in the cage on the morning of
27 September 2015.
Gross Description:
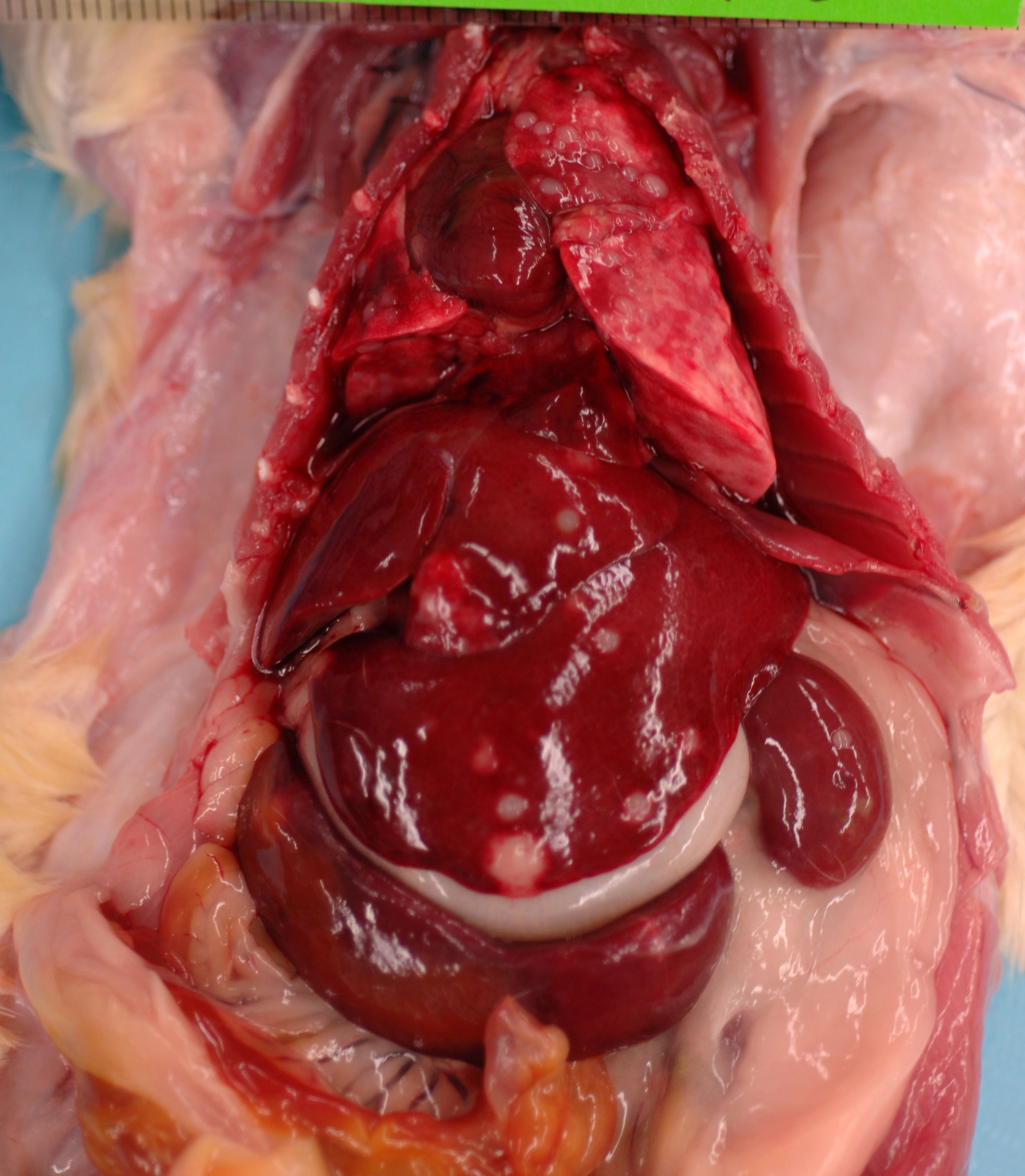
This
689 g, male, CD rat is in optimum body condition with abundant SQ &
visceral fat, 3/5 BCS, & moderate autolysis, especially the GI tract. The
lungs are noncollapsed & pink with multifocal dark red areas & numerous
0.2-0.4 cm, pale, soft, smooth, round, well-demarcated masses affecting all lobes.
The liver has numerous 0.2-0.6 cm, pale, soft, smooth, round, well-demarcated
masses extending into the parenchyma & affecting all lobes. The spleen is
6.5 x 1.5 x 0.5 cm & has a focal, 0.4 x 0.4 x 0.2 cm, pale, soft, smooth,
round, well-demarcated mass. The pancreas appears nodular & firm. The
left kidney has a central, approximately 0.3 x 0.3 cm, slightly raised, pale
area that is wedge-shaped extending into the cortex on cut surface. The right
kidney has 2 similar areas on the caudal pole. The mediastinal & perirenal
lymph nodes are greenish-yellow & enlarged. The urinary bladder is empty.
Histopathologic Description:
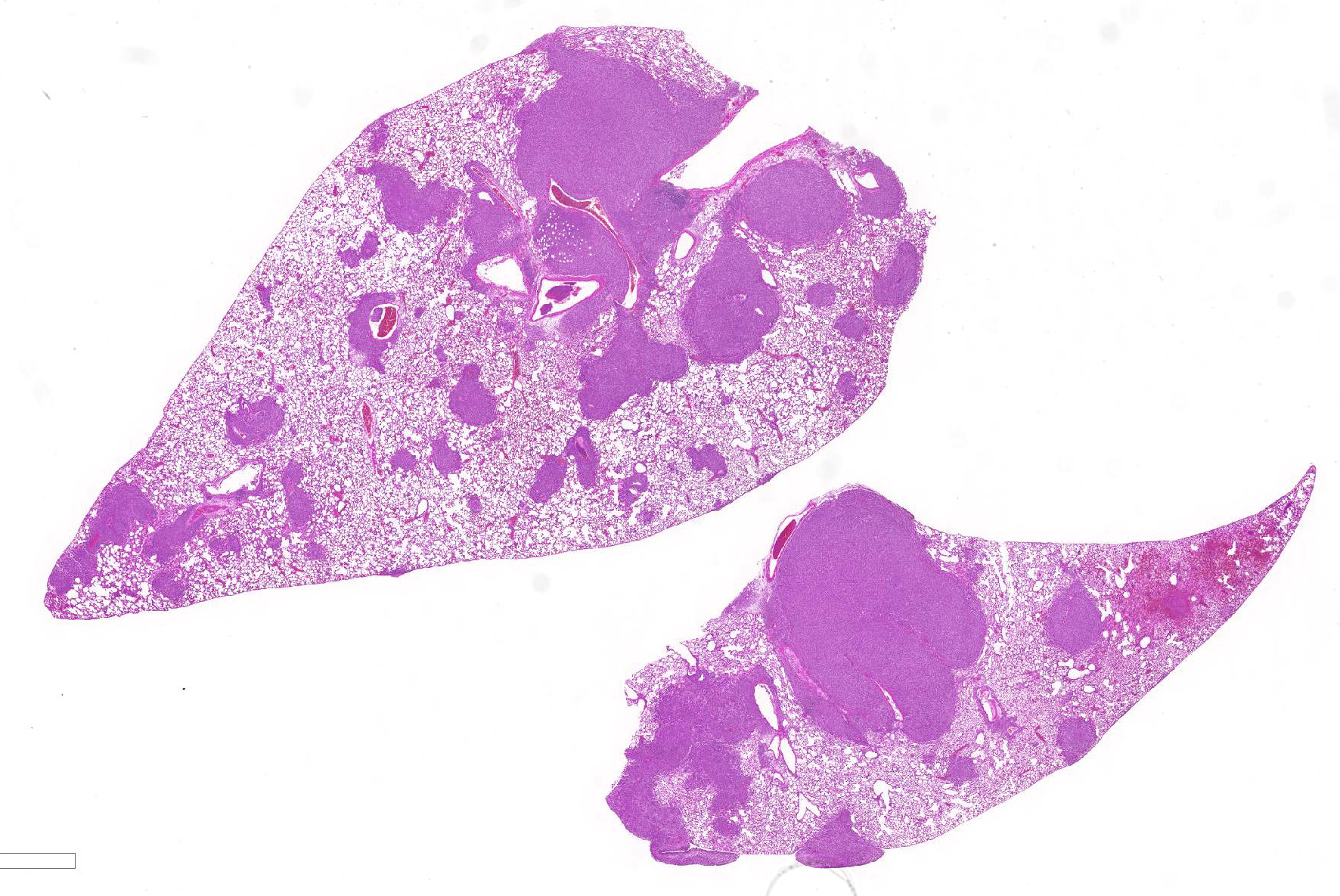
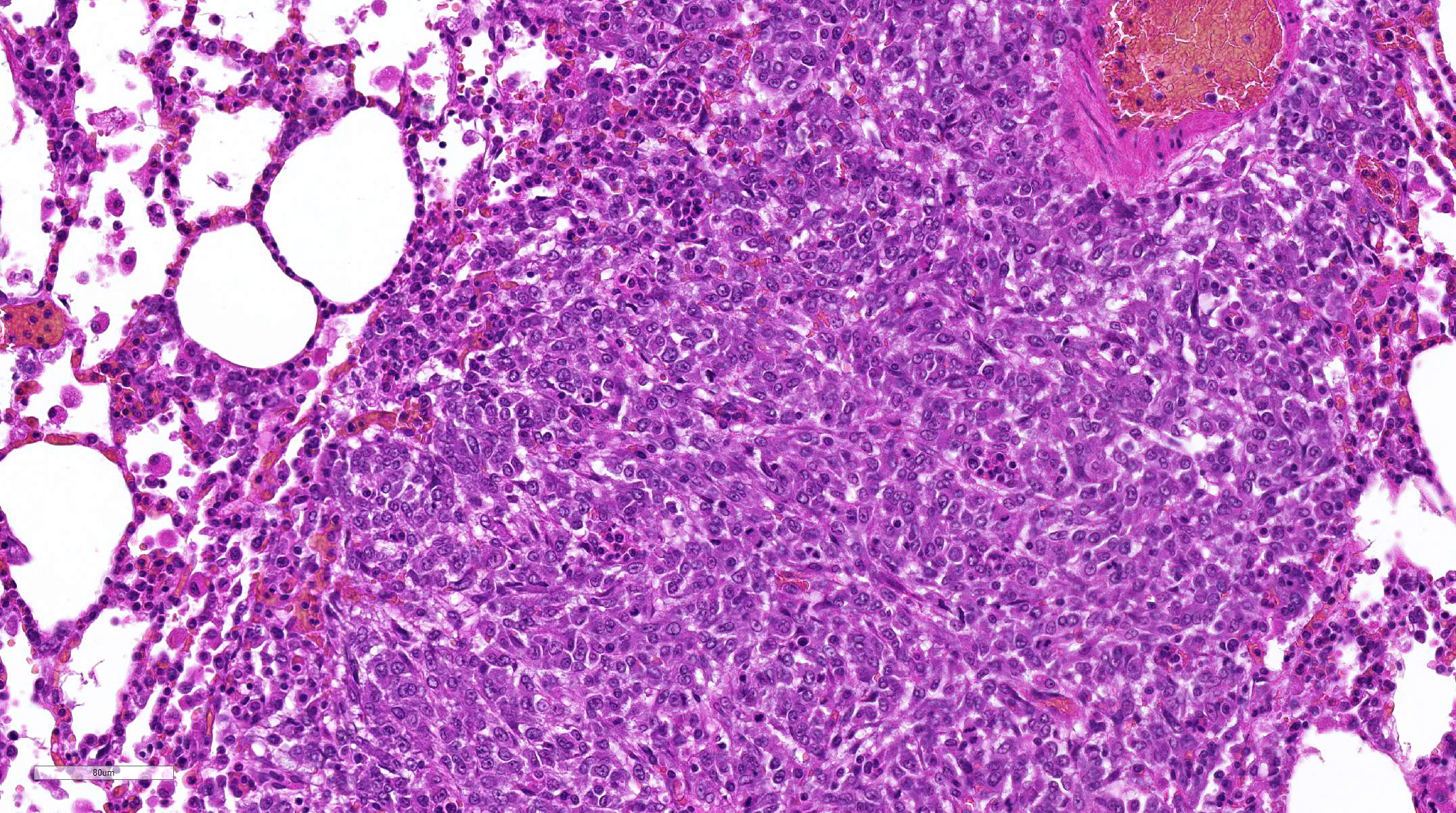
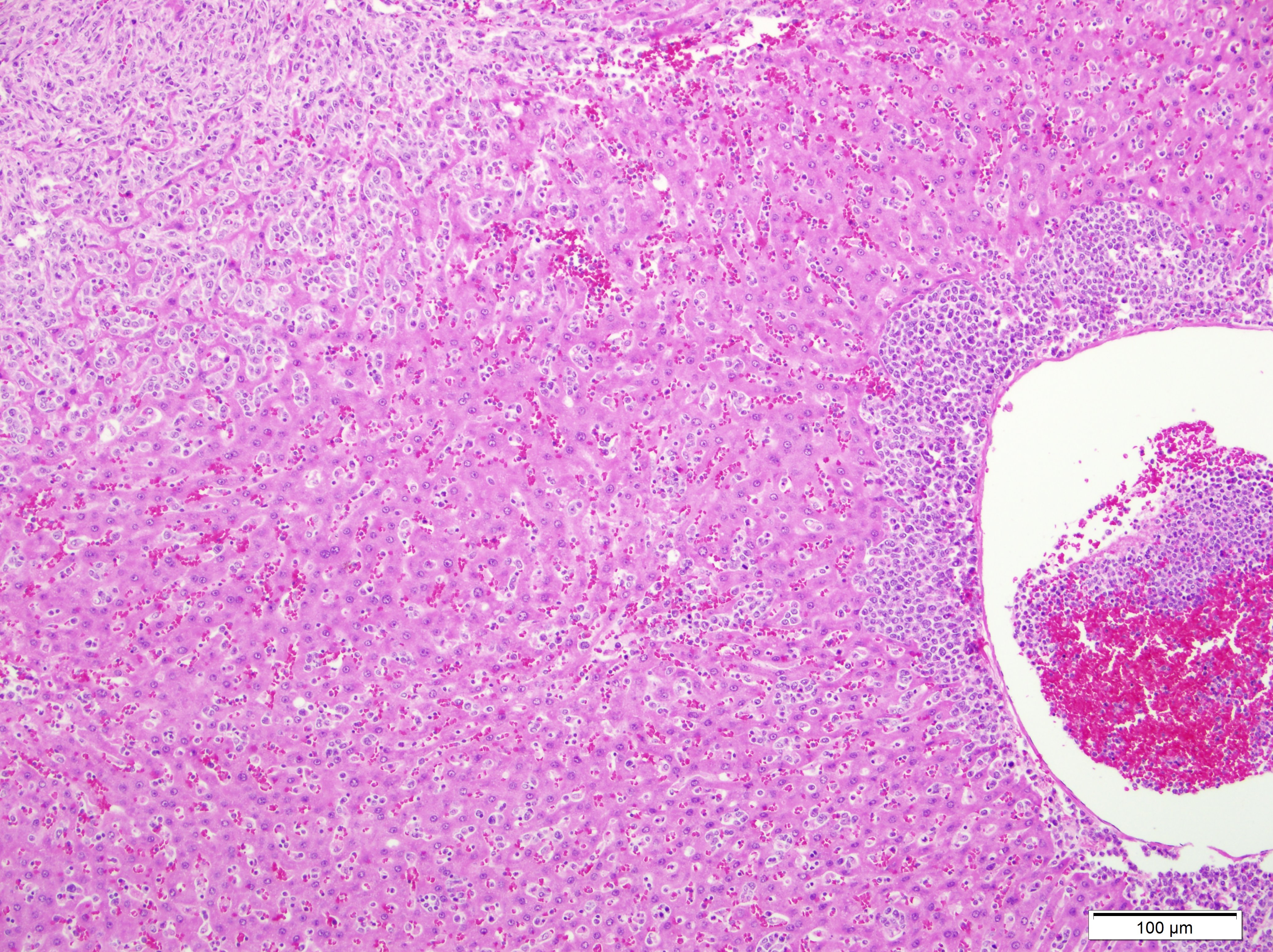
Lung:
Multifocally invading and replacing alveoli, surrounding blood vessels and
rarely filling and replacing bronchioles is an un-encapsulated, poorly
demarcated, cellular neoplasm compose of either round cells arranged in sheets
or less commonly palisading spindle cells all supported by a preexisting
fibrovascular stroma. Neoplastic cells have indistinct cell borders, abundant
pale, eosinophilic cytoplasm, round, oval or reniform nucleus with vesiculate
chromatin and distinct nucleolus. Mitotic rate is 2 per HPF. Multifocally
neoplastic cells are expanding pleura, filling or expanding alveolar septa and
filling blood vessels. Within less affected areas, alveoli often contain
fibrin, hemorrhage, cellular debris and small to moderate numbers of
neutrophils, lymphocytes, and alveolar macrophages. Multifocally individual alveoli
contain small clusters of neoplastic cells. Focally a large bronchiole is
filled with fibrin/cellular debris/mucus admixed with few lymphocytes,
macrophages and sloughed mucosal epithelial cells.
Morphologic Diagnosis:
Lung: Malignant neoplasm, favor histiocytic
sarcoma.
Lab Results:
None
Condition:
Histiocytic sarcoma, rat
Contributor Comment:
Histiocytic
sarcoma in rats is the most common nonlymphoid hematopoietic neoplasm.
6
The incidence in Sprague-Dawleys has been reported to be as low as 1%
6
and as high as 4.7%
8 with no sex predilection. The occurrence of
this neoplasm is seen in animals >12 months of age with one study showing
over 50% of cases in animals in a 18-23 month age group.
7 At
necropsy, sarcomas of this type may be present in the liver, lymph nodes, lung,
spleen, mediastinum, retroperitoneum, or sub-cutaneous
tissue.
1 The most common localizations of this tumor are different
between rat strains and are as follows: the liver and lungs in Sprague-Dawley
rats, liver, bone marrow, lymph nodes, spleen and lungs in Fischer and Donryu
rats and subcutis in Wistar rats.
6 In our case, neoplastic cells
were also seen in the kidney, pancreas, adipose tissue surrounding a number of
the listed organs above as well as within vascular spaces.
JPC Diagnosis:
1.
Lung:
Histiocytic sarcoma, Sprague-Dawley rat,
Rattus norvegicus.
2. Lung:
Pneumonia, interstitial, necrotizing, fibrinonecrotic and eosinophilic,
multifocal, moderate with vascular necrosis.
Conference Comment:
Histiocytic sarcoma (HS) is a relatively common neoplasm in aging
Sprague-Dawley rats, but they have also been reported much less commonly in
other strains (Wistar, Fischer 344, Osborne-Mendels).
1,6,7 Grossly, the
neoplasm is typically pale white to tan, homogenous, and firm forming variably
sized masses that infiltrate and efface normal tissue architecture. HS can
develop at multiple locations, but primary sites are most often in the liver,
lymph nodes, lungs, spleen, bone marrow, retroperitoneum, and the subcutis.
1,6,7,9
Metastatic sites can include any organ, but are most commonly present in the
liver, kidneys, draining lymph node, and lungs.
9 Histologically, HS
have a variable appearance of sheets of large pleomorphic and anaplastic cells
with abundant vacuolated cytoplasm and Langhans-type multinucleated giant cells
or interlacing bundles and streams of elongate palisading fusiform spindle
cells, which is the predominant feature of this case.
1,9
Although
not mentioned by the contributor as a component of this case, conference
participants discussed the frequent association of HS with hyaline droplet (HD)
accumulation in the renal proximal convoluted tubules. In these cases, the
hyaline droplets are immunopositive for lysozyme, a major protein secreted by
macrophages and monocytes.
2-5 There is a direct qualitative
correlation between a droplet accumulation in the kidney and increased tumor
burden in rats. In cases where the HS is confined to a single location, there
may be little to no hyaline droplet accumulation. The widespread metastatic
disease and high tumor burden, described by the contributor, suggests that
there is renal tubule hyaline droplet in this case.
2-5 HD can also
occur in response to a number of other pathologic conditions associated with
accumulation of alpha-2u-globulin in the male rat. These droplets form in the
P2 segment of the proximal tubule and represent secondary lysosomes containing
alpha-2u-globulin bound to a variety of chemicals, including volatile
hydrocarbons, d-limonene, unleaded gasoline, tetra-chloroethylene,
1,3,5-trinitrobenzene, diethylacetylurea, and sodium barbital.
2-5
They have also been observed in chronic progressive nephropathy of rats
associated with accumulation of albumin.
2,4 The HD seen in
tumor-bearing rats are histologically indistinguishable from rats with
alpha-2u-globulin nephropathy. As mentioned above, HD secondary to HS will be
strongly immunopositive for lysozyme, but immunonegative for alpha-2u-globulin
staining; the reverse is true for alpha-2u-globulin nephropathy.
2-5
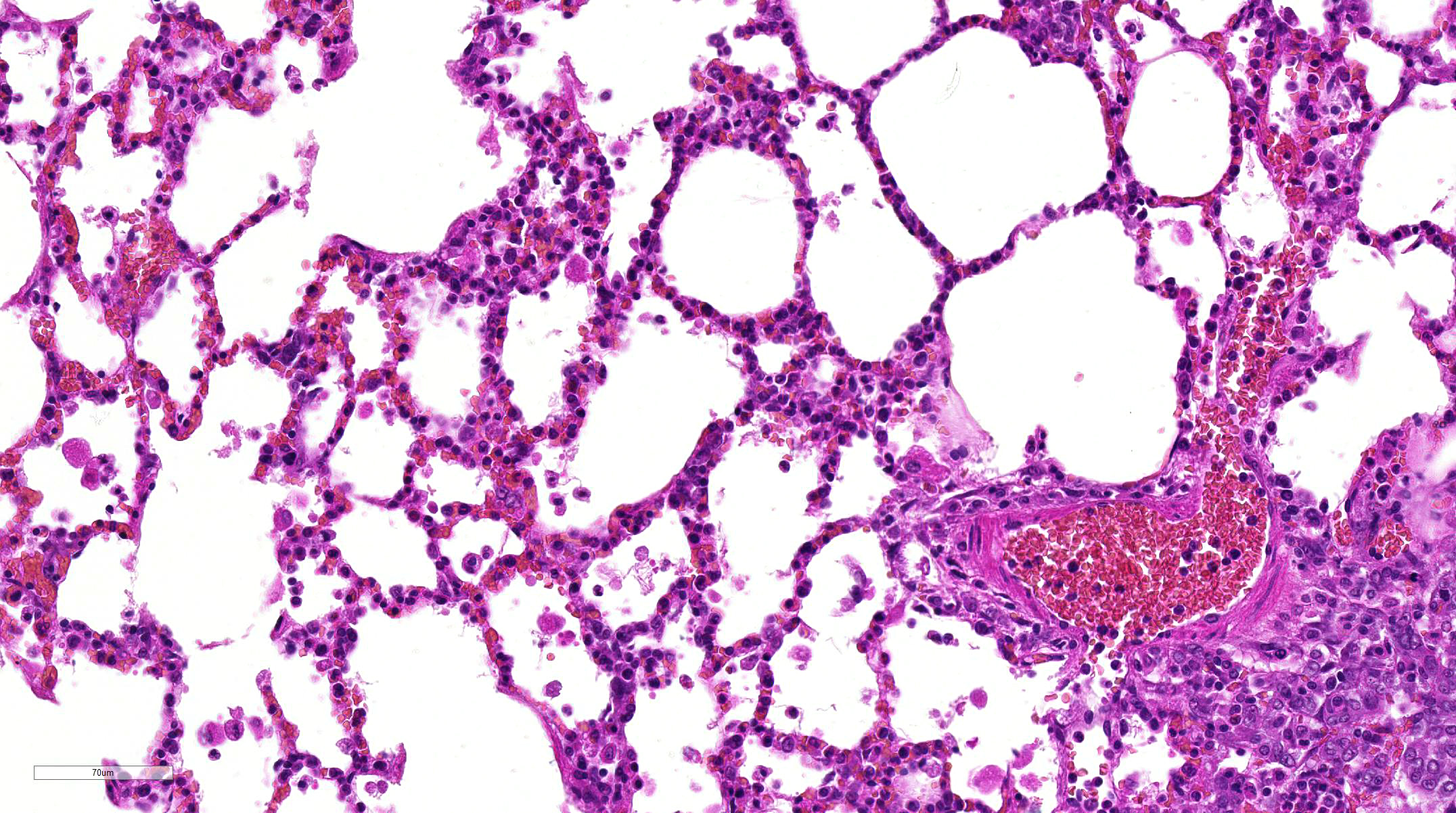
In addition to
multifocal infiltration of HS within this section of lung, conference
participants also noted a moderate infiltration of alveolar macrophages and
eosinophils, predominantly infiltrating multifocal areas of alveolar septal
thickening and necrosis. Accumulation of inflammatory cells is not uncommon in
pulmonary neoplastic disease, especially HS
9; however, conference
participants could not explain the widespread presence of eosinophils within
areas of inflammation and necrosis. It is possible that the inflammation
present in this case may be related to the administration of the test article
rather than the neoplasm; although, the nature of the test article is not
specified by the contributor.
References:
1.
Barthold SW, Griffey SM, Percy DH. Rat. In: Pathology of laboratory
rodents and rabbits. 4th ed. Ames, IA: John Wiley & Sons, Inc.;
2016:167.
2.
De Rijk EPCT, Ravesloot WM, et al. A fast histochemical
staining method to identify hyaline droplets in the rat kidney. Toxicol
Pathol. 2003; 31:462-464.
3.
Frith CH, Ward JM, and Chandra M. The morphology,
immunohistochemistry, and incidence of hematopoietic neoplasms in mice and
rats. Toxicol Pathol. 193; 21:06218. 1993.
4.
Hard GC. Some aids to histological recognition of hyaline
droplet nephropathy in ninety-day toxicity studies. Toxicol Pathol.
2008; 36:1014-1017.
5.
Hard GC, Snowden RT. Hyaline droplet accumulation in rodent
kidney proximal tubules: An association with histiocytic sarcoma. Toxicol
Pathol. 1991; 19:88-99.
6.
Kemmochi Y, Takahashi A, Miyajima K, Yasui Y, Tanoue G, Shoda
T, Kakimoto K. Spontaneous histiocytic sarcoma of the popliteal lymph node in a
young Sprague-Dawley rat. J Toxicol Pathol. 2010; 23:161-164.
7.
Ogasawara H, Mitsumori K, Onodera H, Imazawa T, Shibutani M,
and Takahashi M. Spontaneous histiocytic sarcoma with possible origin from the
bone marrow and lymph node in Donryu and F-344 rats. Toxicol Pathol.
1993; 21:6370.
8.
Squire RA, Brinkhous KM, Peiper SC, Firminger HI, Mann RB,
and Strandberg JD. Histiocytic sarcoma with a granuloma-like component
occurring in a large colony of Sprague-Dawley rats. Am J Pathol. 1981;
105: 2130.
Valli VEO, Kiupel M, Bienzle D. Hematopoeitic system. In Maxie MG, ed.
Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 3. 6th ed.
Philadelphia, PA: Elsevier Ltd; 2016:250-255.