Signalment:
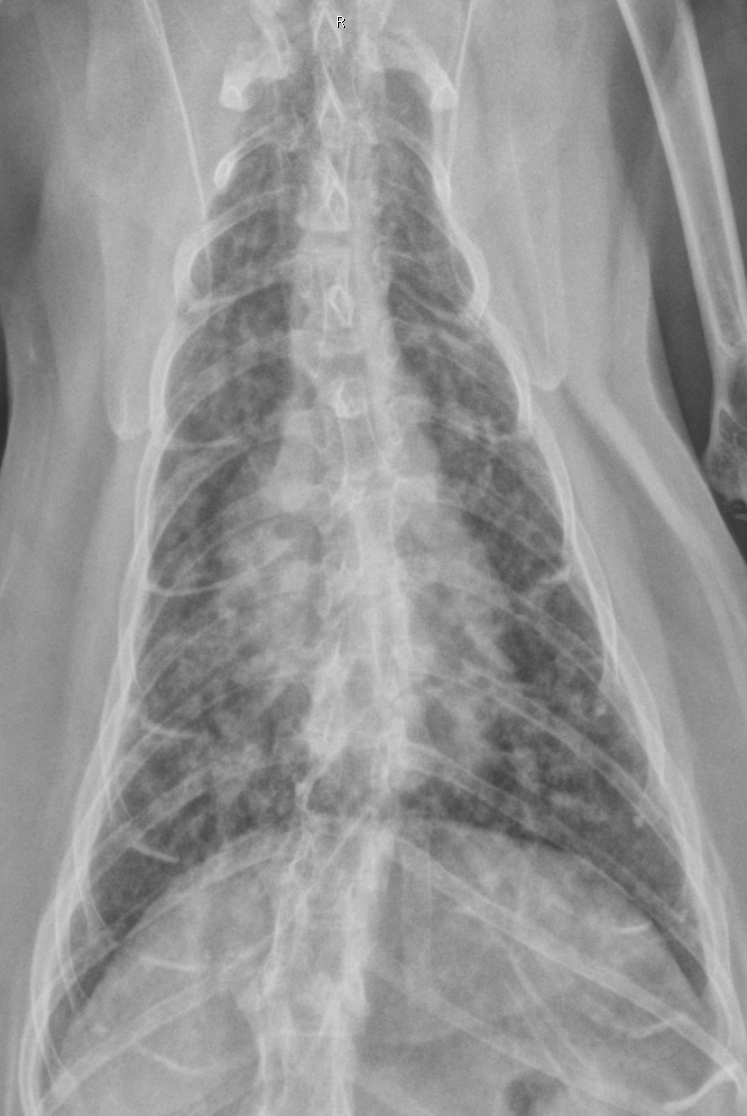
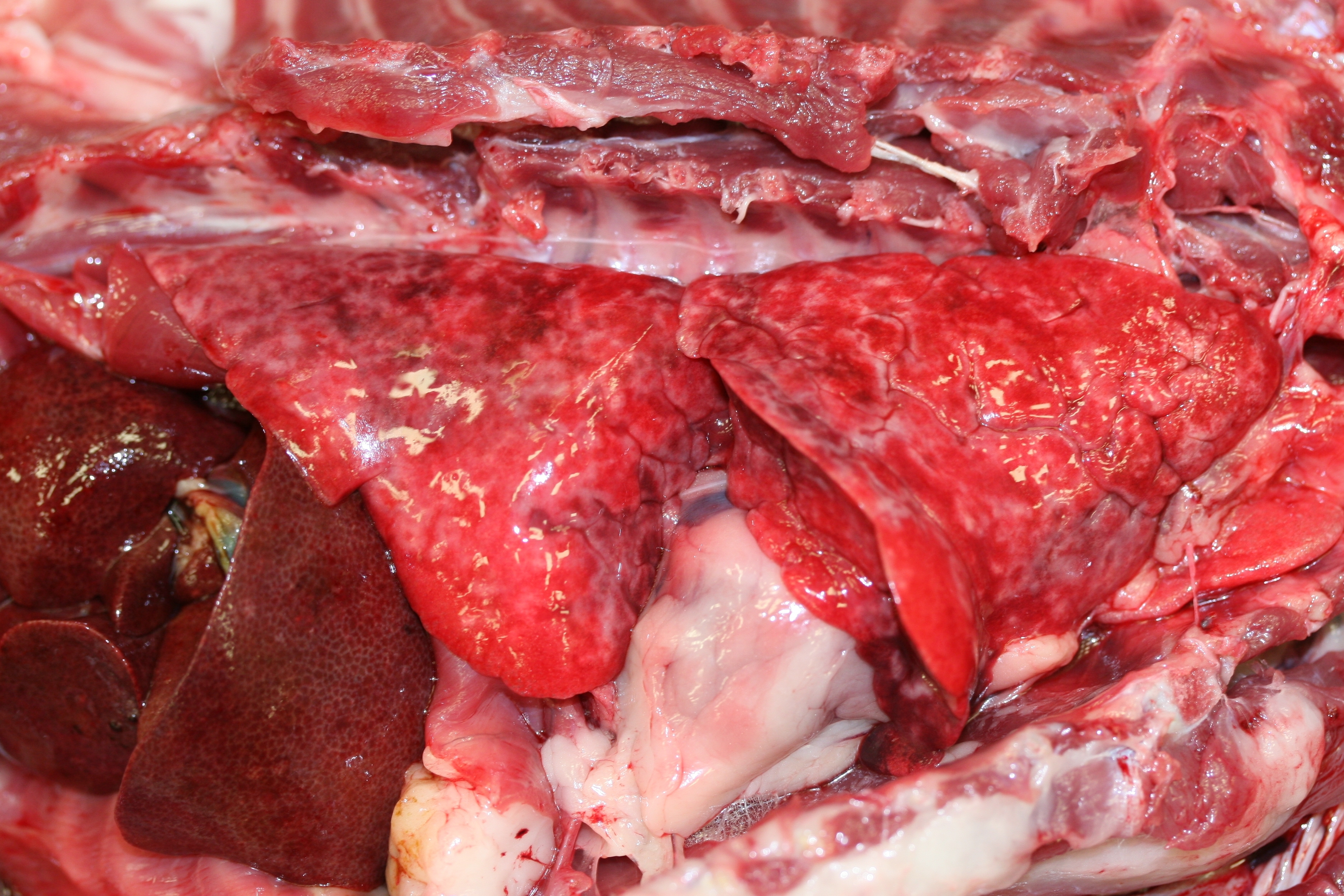
Gross Description:
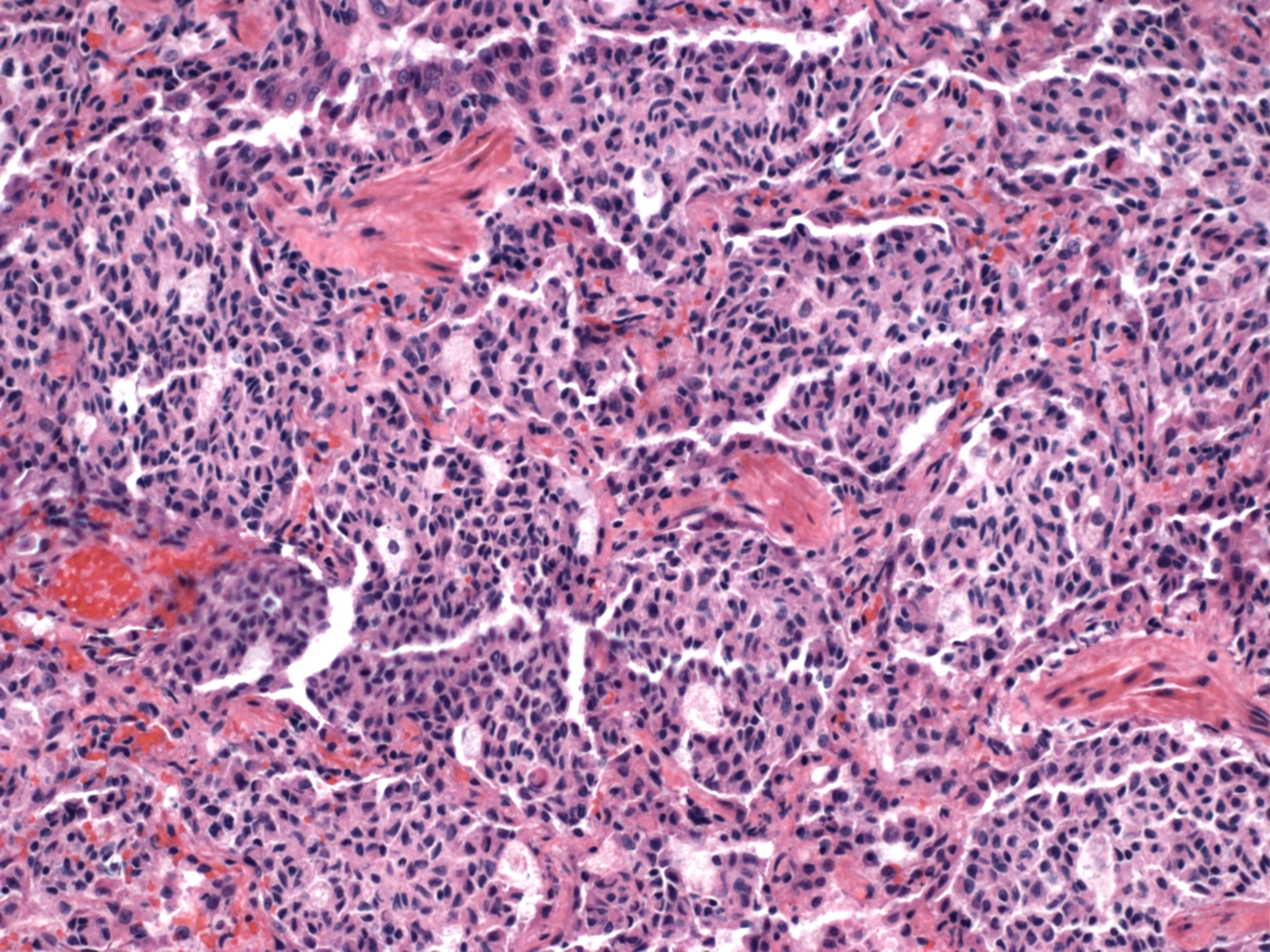
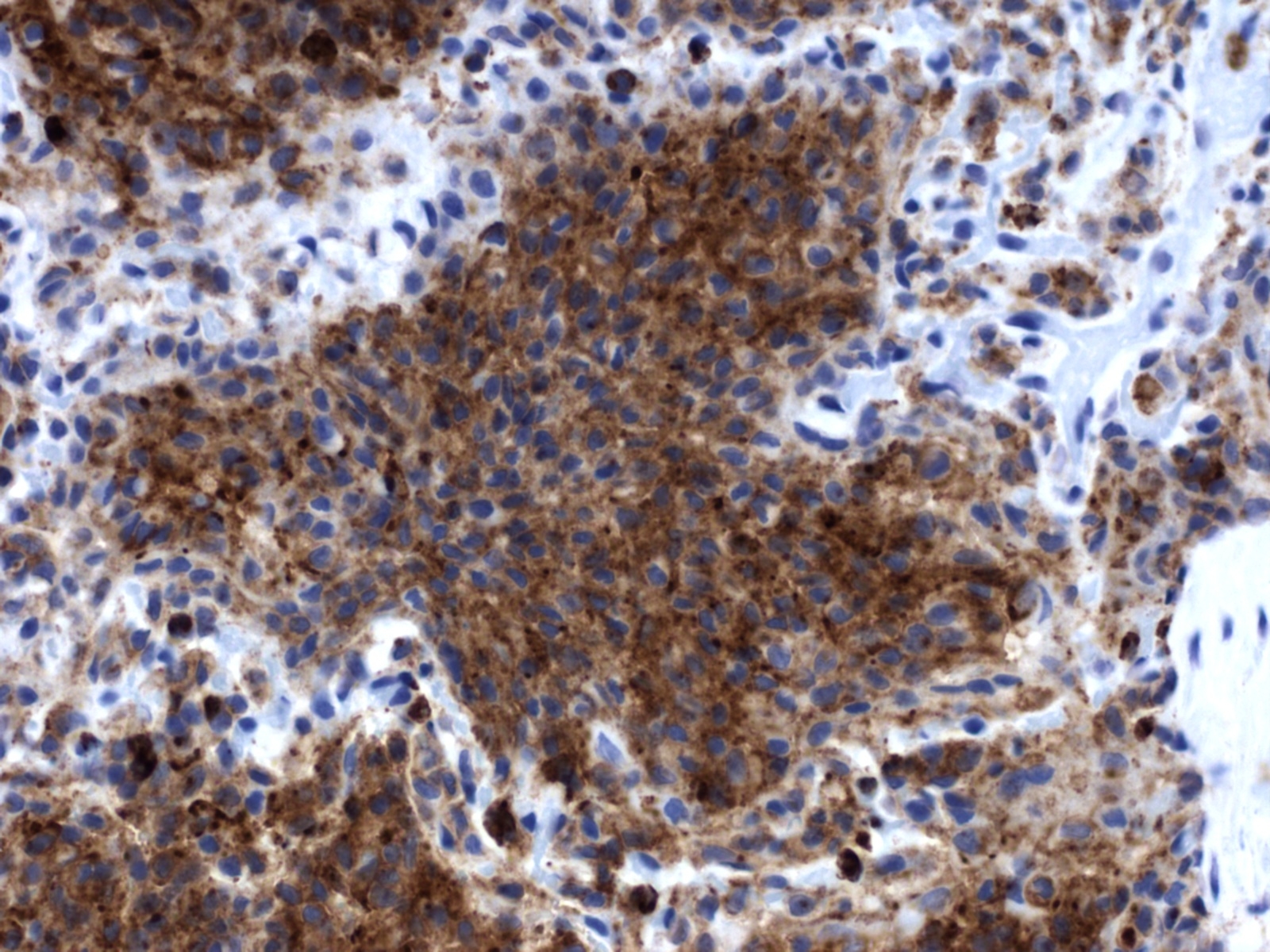
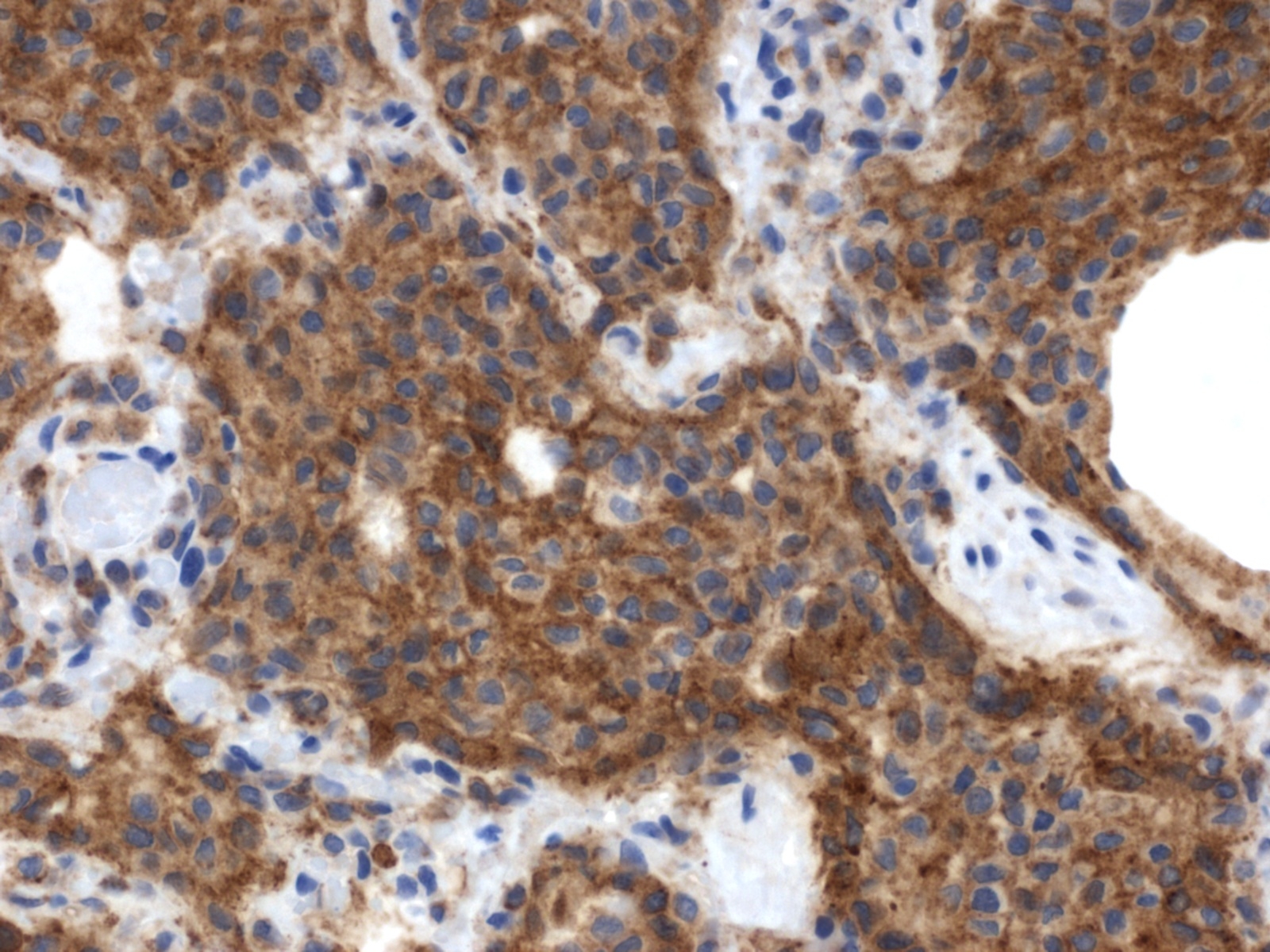
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
In the corresponding human interstitial lung disease, which occurs primarily in young adult cigarette smokers, PLCH was found to be the result of mixed clonal and nonclonal expansion of nonmalignant Langerhans cells that arises in a setting of Langerhans cell hyperplasia.(11) This, along with the fact that it is frequently associated with clinical regression after steroid therapy and cessation of smoking, supports the view that PLCH represents a reactive disorder rather than a neoplastic process. It occurs in a slightly higher percentage in women and is frequently found only in the lungs, although multiorgan involvement may also occur.(9,10) PLCH represents one of a spectrum of Langerhans cell proliferative diseases (Langerhans cell histiocytosis), which occur more frequently in children and are marked by proliferation and infiltration of various organs by Langerhans cells.(8,10) In addition to the lung, the organs most commonly affected by Langerhans cell histiocytosis include bone and skin, although any organ can be affected. In the recent report of PLCH in 3 cats, affected organs included the lung, pancreas, kidney, liver, and various lymph nodes.(3) Extrapulmonary involvement was not observed in the present case. A characteristic feature of PLCH, in addition to the marked proliferation of alveolar histiocytes, is the presence of rod-shaped Birbeck granules, the hallmark organelle of the Langerhans cell, within the cytoplasm of lesional histiocytes when viewed with transmission electron microscopy.
JPC Diagnosis:
Conference Comment:
Reports of histiocytic diseases of the cat are few and limited to feline progressive histiocytosis,2 feline pulmonary Langerhans histiocytosis,(3) histiocytic sarcoma(7) and hemophagocytic histiocytic sarcoma;(4) the cell type in the first two conditions is of Langerhans cell lineage, dendritic cell origin for the third while the findings in the last entity are most consistent with macrophage origin. In the feline progressive histiocytosis and feline pulmonary Langerhans histiocytosis, it remains uncertain whether the conditions represent a reactive or neoplastic process. In contrast to cats, histiocytic diseases in the dog are much more common, and the nature of the conditions as reactive or neoplastic are better characterized. The classifications and corresponding cell of origin are as follows: reactive cutaneous/systemic histiocytosis (interstitial dendritic cell); cutaneous histiocytoma (Langerhans cell); local and disseminated histiocytic sarcoma (myeloid dendritic cell); and hemophagocytic histiocytic sarcoma (macrophage). (1,5,7)
Development of dendritic cells, Langerhans cells and macrophages begins with CD34+ progenitor cells in the bone marrow, and further differentiation produces three subsets of cells: CD34+/CLA+ (cutaneous lymphocyte antigen); CD34+/CLA-; and CD34+/IL-3Rα-rich cells.(1) The CD34+/CLA+ and CD34+/CLA- cells, under the influence of stem cell factors (GM-CSF and TNF-α) differentiate into CD1+/CD14- and CD1a-/CD14+ cells, respectively. Again, under the influence of GM-CSF and TNF-α, CD1a-/CD14+ cells differentiate into interstitial dendritic cells, or, if stimulated by M-CSF, undergo differentiation to macrophages. The CD1+/CD14- subtypes differentiate into Langerhans cells under the influence of GM-CSF, TNF-α, and TGF-β. The CD1a/CD14+ subtypes can also arise from CD14+ blood monocytes under the influence of GM-CSF and IL-4. Myeloid dendritic cells arise from CD34 +/IL-3Rα-rich cells when stimulated by IL-3 and GM-CSF.(1)
The immunophenotyping of the cells comprising the histiocytic diseases varies based on the reference text or journal consulted, and reflects the continuous information explosion in this very active field of research. After review of the literature and the veterinary reference text of Jubb, Kennedy and Palmers Pathology of Domestic Animals, the following list outlines the most consistent and commonly cited immunophenotypes:(1-7)
- Langerhans cells: MHC II; CD1a, c; CD11c; CD18; langerin; ICAM-1; and E-cadherin positive
- Interstitial dendritic cells: MHC II; CD1c; CD4; CD11b, c; CD18; CD90 (Thy-1) positive
- Myeloid dendritic cells: MHC II; CD1; CD11c; ICAM-1; -� CD90 positive
- Macrophage: MHC II; CD11d/CD18; β2-integrin; -� CD11c/CD18 and CD1c positive
Readers are encouraged to review Wednesday Slide Conference 24, Case IV, 2008-2009, for a thorough review of canine histiocytic diseases.
References:
2. Affolter VK, Moore PF. Feline progressive histiocytosis. Vet Pathol. 2006;43:646-655.
3. Busch MDM, Reily CM, Luff JA, Moore PF. Feline pulmonary Langerhans cell histiocytosis with multiorgan involvement. Vet Pathol. 2008;45(6):816-824.
4. Friedrichs KR, Young KM. Histiocytic sarcoma of macrophage origin in a cat: case report with a literature review of feline histiocytic malignancies and comparison with canine hemophagocytic histiocytic sarcoma. Vet Clin Pathol. 2008;37(1):121-128.
5. Fulmer AK, Mauldin GE. Canine histiocytic neoplasia: an overview. Can Vet J. 2007;48:1041-1050.
6. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed., vol. 1. Philadelphia, PA: Elsevier Ltd; 2007:768-770.
7. Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Histiocytic Sarcoma. In: Skin diseases of the dog and cat, clinical and histopathologic diagnosis. 2nd ed., Ames, Iowa: Blackwell Science Ltd; 2005:848-852.
8. Moore PF, Affolter VK, Vernau W. Canine hemophagocytic histiocytic sarcoma: a proliferative disorder of CD11d+ macrophages. Vet Pathol. 2006:43:632-645.
9. Satter, EK. High WA. Langerhans cell histiocytosis: a review of the current recommendations of the histiocyte society. Pediatr Dermatol. 2008;25(3)291-295.
10.Vassalo, R, Ryu JH. Pulmonary Langerhans cell histiocytosis. Clin Chest Med. 2004;25(3):561-571.
11.Vassalo, R, Ryu, JH, Colby TV, et al. Pulmonary Langerhans-cell histiocytosis. N Engl J Med. 2000;342(26): 1969-1978.
12.Yousem SA, Colby TV, Chen YY, et al. Pulmonary Langerhans cell histiocytosis: molecular analysis of clonality. Am J Surg Pathol. 2001;25(5):630-636.3.