Signalment:
Gross Description:
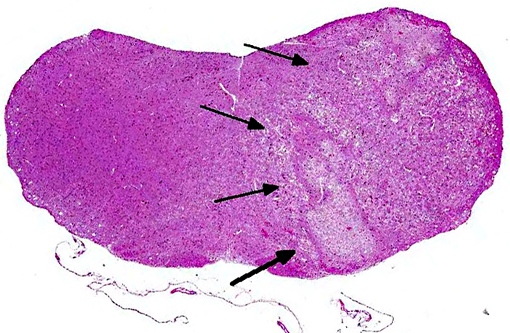
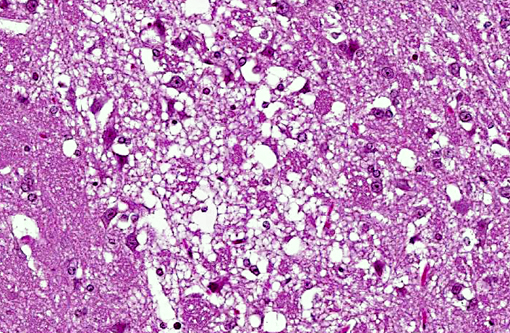
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
This lesion has been previously reported by Southard and Brayton in Swiss ICR/CD-1 mice as spontaneous vestibular syndrome and spontaneous unilateral brainstem infarction in Swiss mice.(11) Spontaneous vestibular syndrome in this outbred mouse stock is thought to be secondary to occlusion or dissection of the extracranial or intracranial vertebral artery and/or its branches.Â
The paired extracranial vertebral arteries arise from the subclavian artery, or rarely, directly from the aortic arch. The largest branch of the intracranial vertebral artery supplies the dorsal medulla and cerebellum and is termed the posterior inferior cerebellar artery (PICA). At the base of medulla oblongata the two intracranial vertebral arteries merge at the midline to form the basilar artery which then branches to form the posterior cerebral arteries and the posterior communicating arteries comprising the Circle of Willis.(1-3)
The clinical and histopathologic features of this syndrome in Swiss ICR/CD-1 mice have been compared by Southard and Brayton to a constellation of clinical signs seen secondary to occlusion or dissection of the intracranial vertebral artery or posterior inferior cerebellar artery in humans termed Wallenberg syndrome or lateral medullary syndrome.(9,10,12) However, these Swiss mice do not share the most common risk factors associated with stroke in humans, including hypertension, diabetes mellitus, smoking, and hyperlipemia leading to atherosclerosis(1,6,8) (in humans this constellation of clinical signs is sometimes also attributed to neoplasia involving the brainstem(12)). Currently, the underlying etiology in these Swiss mice, such as an underlying congenital intravertebral artery stenosis, is not known and deserves further investigation. Vertebral artery occlusions leading to medullary infarction in humans are associated with both gender and race predilections. Intracranial vertebral artery occlusions or dissections are more common in women and people of African and Asian descent, while extracranial vertebral artery occlusions or dissections are more common in white males.(1) Medullary infarctions are further classified as lateral medullary infarctions (LMI, Wallenberg, or Wallenbergs syndrome) and medial medullary infarctions (MMI or Dejerine syndrome).(4,5,7) The major symptoms associated with LMI include sensory disturbances affecting the face, dysarthria, vertigo, Horners syndrome, cerebellar ataxia, and decreased pharyngeal reflexes. The major symptoms associated with MMI include motor weakness and sensory disturbances of the extremities, however, the clinical signs of LMI and MMI commonly overlap depending on the areas and extent of the brainstem affected.(4,5) It is not surprising that the vascular supply to the medial and lateral medulla differs (and sometimes varies). As mentioned above, the lateral medulla receives its arterial supply from the intracranial vertebral artery and PICA, while the upper and lower medial medulla receives arterial blood from the anteromedial medullary arteries. These arise from the intracranial vertebral artery in the upper medulla and from the anterior spinal artery in the lower medulla.(5) The reported prevalence of medullary infarctions in humans varies widely and is continually changing owing to the development and widespread use of magnetic resonance imaging as a diagnostic tool, which has improved anatomic localization of the lesions. The incidence of spontaneous medullary infarction in Swiss ICR/CD-1 mouse is not known.Â
JPC Diagnosis:
Conference Comment:
A Laxol Fast Blue (LFB) stained virtual slide is available here.
References:
2. Caplan LR. The intracranial vertebral artery: a neglected species. The Johann Jacob Wepfer Award 2012. Cerebrovasc Dis. 2012;34:20-30.
3. Cloud GC, Markus HS. Diagnosis and management of vertebral artery stenosis. QJM. 2003;96:27-54.
4. Fukuoka T, Takeda H, Dembo T, Nagoya H, Kato Y, Deguchi I, et al. Clinical review of 37 patients with medullary infarction. J Stroke Cerebrovasc Dis. 2012;21:594-599.
5. Kameda W, Kawanami T, Kurita K, Daimon M, Kayama T, Hosoya T, et al. Lateral and medial medullary infarction: a comparative analysis of 214 patients. Stroke. 2004;35:694-699.
6. Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, et al. Effects of dietary sodium and genetic background on angiotensinogen and Renin in mouse. Hypertension. 2002;39:1007-1014.
7. Lee MJ, Park YG, Kim SJ, Lee JJ, Bang OY, Kim JS. Characteristics of stroke mechanisms in patients with medullary infarction. Eur J Neurol. 2012;19:1433-1439.
8. Lemini C, Jaimez R, Franco Y. Gender and inter-species influence on coagulation tests of rats and mice. Thromb Res. 2007;120:415-419.
9. Razak A, Clark D, Farooq MU, Kassab MY. Wallenberg's syndrome with extradural-extracranial origin of the posterior inferior cerebellar artery. Neurol Sci. 2011;32:711-713.
10. Sameshima T, Morita A, Yamaoka Y, Ichikawa Y. Ipsilateral sensorimotor deficits in lateral medullary infarction: a case report. J Stroke Cerebrovasc Dis. 2012.
11. Southard T, Brayton CF. Spontaneous unilateral brainstem infarction in Swiss mice. Vet Pathol. 2011;48:726-729.
12. van den Bergh P, Dom R. Wallenberg's syndrome caused by a craniopharyngioma "en plaque". J Neurol. 1983;229:61-64.