Signalment:
Gross Description:
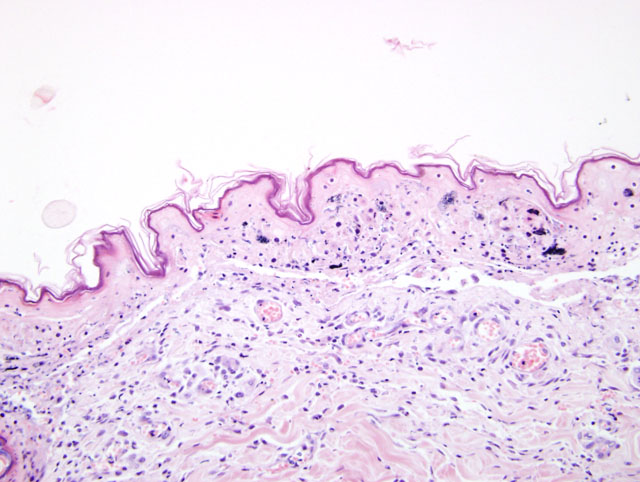
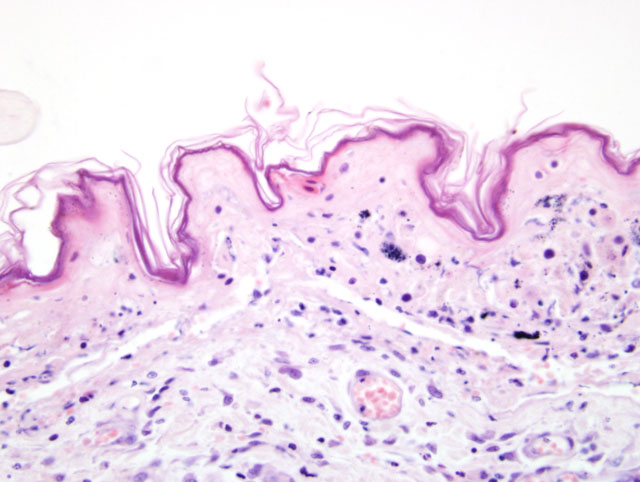
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Sodium: 148 mEq/L (142-151)
Potassium: 3.1 mEq/L (3.9-5.3)
Chloride: 115 mEq/L (107-117)
Bicarb: 19 mEq/L (15-25)
Anion gap: 17 mEq/L (13-25)
Na: K: 48
Urea: 40mg/dL (8-30)
Creat: 1.7mg/dL (0.5-1.3)
Calcium: 9.1 mg/dL (9.3-11.6)
Phosphate: 6.4 mg/dL (2.8-5.3)
Magnes: 2.8 mEq/L (1.4-2)
Tot Prot: 4.3 g/dL (5.6-7.1)
Alb: 2.1 g/dL (3.1-4.1)
Glob: 2.2 g/dL (1.9-3.6)
A/G: 0.95
Glucose: 183 mg/dL (60-120)
ALT/P5P: 85 U/L (25-106)
AST/P5P: 120 U/L (16-50)
Alk Phos: 348 U/L (12-122)
GGT: <3 U/L (0-10)
TotBili: 5 mg/dL (0-0.3)
Dir Bili: 4 mg/dL (0-0.1)
Ind Bili: 1 mg/dL (0-0.3)
Amylase: 934 U/L (286-1124)
Cholesterol: 269 mg/dL (124-335)
CK: 3633 U/L (58-241)
Iron: 93 ug/dL (98-220)
TIBC: 213 ug/dL (249-496)
%SAT: 44% (28-62) Hematology
HCT: 34% (42-57)
HB: 11.5 g/dL (14.6-19.7)
RBC: 4.6 mill/uL(6.1-8.5)
MCV: 73 fL (63-74)
MCH: 25 pg (21-26)
MCHC: 34 g/dL (32-37)
RDW: 12.8% (11.3-14)
Retic: 0.4% (0.2-1.1)
Retic-abs: 18.4 thou/ul (10.1-75.9)
Nucl RBC: 1/100WBC (0-1/100WBC)
WBC: 24.8 thou/ul (6.2-14.4)
Seg Neuts: 19.1 thou/ul (3.4-9.7)
Band Neuts: 2.5 thou/ul (0-0.1)
Lymph: 0.5 thou/ul (1.2-4.7)
Mono: 0.7 thou/ul (0.1-1)
Eosin: 2 thou/ul (0.1-2)
Baso: 0 thou/ul (0-0.1)
Plat smear: Low
Plat: 67 thou/ul (179-483)
MPV: 12.3 fL (8.4-13.2)
TP: 6.6 g/dL (5.9-7.8)
RBC Morphology: No significant abnormalities
WBC Exam: Toxic changes in neutrophils (moderate)
Plasma appearance: Icterus (moderate) Coagulation Panel
Activated Partial Thromboplastin Time: 21.5s (10-17)
Antithrombin 3: 48% (75-120)
D-dimer: 250-500 ng/ml (<250)
Fibrinogen: 1414 mg/dL (150-480)
Protein C: 70%
Prothrombin Time: 17s (14-18)
Thrombin Clotting Time: 5s (5-9) Ancillary Tests
Antinuclear antibody test: Negative
Condition:
Contributor Comment:
Clinically, TEN represents the most severe of the three syndromes.3,4,11 This distinctive clinical presentation has led many to believe this is a pathologically distinct entity form EM.3,4,11 Microscopically, TEN is characterized by extensive, full thickness, epidermal cell death (fig. 1-1, 1-2). In the classical sense, this is associated with little to no inflammation. However more advanced cases can develop a degree of inflammation, particularly following dermal-epidermal detachment.3 SJS bears similarity to TEN, though has less extensive epidermal detachment.5
Many authors now consider EM to be clinically and histologically distinct form SJS and TEN.3,4 Classically, EM is characterized by multiple -�-�target lesions (present in 15.9% of cases)11, and these affect less than 50% of the body. The severity of disease can be variable, but removal of the inciting causes usually produces a clinical resolution within three weeks.11 Histologically, EM is characterized by multi-level, single cell-death surrounded by lymphocytes or macrophages (satellitosis). Severe cases of EM (EM-major) can develop to transepidermal necrosis, whereby lesions may resemble those of TEN.
A diagnosis of EM, with confluent areas of epidermal necrosis, was considered as a differential in this case. However, we feel that the clinical and histologic findings differ from EM on a number of levels. Importantly, the clinical disease in this animal was severe and rapidly progressive: dermatologic lesions affected large areas of the body (significantly greater than 50%) and there were large areas of epidermal detachment. This was accompanied by profound depression and lethargy, with vomiting and diarrhea. Blood biochemistry and hematology also revealed electrolyte abnormalities; a coagulation panel revealed late stage changes consistent with Disseminated Intravascular Coagulation. Although animals with EM can also be systemically unwell, the extent of ante-mortem lesions would favor a diagnosis of TEN. From a histologic perspective, the lymphocytes could represent a more chronic change, and possibly a sequel to the extensive epidermal changes.
All of the above syndromes have been associated with an immune reaction against a hematogenous antigen. Drug administration appears to be an important predisposing factor, particularly in cases of TEN where there was a temporal association in over 80% of human cases.4,11 A similar association seems to exist in the dog.5 Medication have also been associated with EM and SJS: however the study by Hinn et al. found that unlike TEN, the majority of EM cases were not associated with previous drug administration. Other antigens such as infections, vaccination, food, and neoplasms are therefore thought to account for disease in animals.11 The remaining cases are thought to be idiopathic.
Given the infrequency of the disease in veterinary species, recent studies regarding pathogenesis are largely derived from the human literature. It is generally agreed that the disease represents massive, immune mediated apoptosis of epidermal keratinocytes; however the mechanism by which this occurs is controversial. A number of different theories have been proposed. Some papers have suggested a primary role for both T-cells and NK cells in the development of disease.2,8,10 Part of the basis for this is the presence of CD8+ lymphocytes within blister fluid and the epidermis in the early stages of disease. Blister fluid is also found to contain high levels of soluble IL-2 receptor, indicative of activated T-cells.10 Other cytokines and chemokines may also play a role in human cases of SJS and TEN.2,8,10 In particular, over expression of TNF-_ has been shown in many cases of SJS/TEN.10 IL-5, IL-13 and IFN-γ, may also contribute to the inflammation.2
A primary role of apoptotic mechanisms has also been proposed.1,7,12,13 The activation of Fas (CD95), through binding of FasL, may be fundamental to the development of disease. Viard et al. showed that blocking Fas/FasL interactions, through the antibodies in pooled human sera, could prevent keratinocyte death in vitro.13 In one report, a single dog with SJS was successfully treated with human immunoglobulin, and the authors attributed this to the same mechanism.9 Initial studies suggested that FasL was translocated to the surface of affected keratinocytes. However subsequent publication have failed to consistently demonstrate surface expression of FasL in affected patients.1 One study found increased levels of soluble Fas ligand (sFasL) in the peripheral blood of patients with SJS-TEN, and this was derived form peripheral blood mononuclear cells(PBMCs).1 In this same study, keratinocyte apoptosis could be induced in vitro, following treatment with serum from SJS and TEN patients. Single nucleotide polymorphisms in Fas and FasL have been suggested to confer susceptibility to these diseases in humans.7
Following the appropriate stimulation, apoptosis may occur via activation of death receptors, of which Fas is the most widely studied.6 In the Fas pathway, binding with FasL (from either autocrine, paracrine or endocrine route) induces receptor triimerization. This facilitates binding of a Fas-associated death domain (FADD), leading to conversion of procaspase 8 and subsequent caspase activation. Other potential mechanisms of apoptosis include activation of TNF-related apoptosis-inducing ligand (TRAIL), which may be important given the high concentrations of TNF-_ in lesions.10 Activated cytotoxic T-cells may also induce apoptosis by perforin/granzyme B.10 The contribution of these various pathways is unclear, however there is evidence to suggest that multiple pathways may be involved.
JPC Diagnosis:
Conference Comment:
In general the separation of these disease entities depends on the clinical as well as the histological criteria. Below are listed general characteristics of EM, SJS, and TEN.4
| Condition | Location | Characteristics |
| EM, Minor | No signs of systemic illness At least 1 mucosal surface affected < 10% of body surface | Lymphohistiocytic, perivascular and interface Lymphocytic sattelitosis |
| EM, Major | Signs of systemic illness >1 mucosal surface affected 10-50% of body surface <10% epithelial detachment | High degree of epidermal inflammation, vesiculobullous lesions |
| Steven-Johnson Syndrome | 50% of body surface 10-30% epithelial detachment | Severe epithelial necrosis |
| TEN | Generalized disease More than 30% epithelial detachment | None, or minimal except when ulcerated |
References:
2. Caproni M, Torchia D, Schincaglia E, Volpi W, Frezzolini A, Schena D, Marzano A, Quaglino P, De Simone C, Parodi A, Barletta E, Fabbri P: Expression of cytokines and chemokine receptors in the cutaneous lesions of erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol 155:722-728, 2006
3. Ginn PE, Mansell JEKL, Rakich PM: Skin and appendages. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1, pp. 656-658. Elsevier Saunders, Philadelphia, PA, 2005
4. Gross TL, Ihrke PJ, Walder EJ, Affolter VK: Necrotizing diseases of the epidermis. In: Skin Diseases of the Dog and Cat, 2nd ed., pp. 65-68, 75-78, 80-84. Blackwell, Ames, IA, 2005
5. Hinn AC, Olivry T, Luther PB, Cannon AG, Yager JA: Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis in the dog: clinical classification, drug exposure and histopathological correlations. J Vet Allergy Clin Immunol 6:13-20, 1998
6. Kumar V, Abbas AK, Fausto N: Cellular adaptations, cell injury, and cell death. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas, AK, Fausto N, 7th ed., pp. 26-32. Elsevier Saunders, Philadelphia, PA, 2005
7. Murata J, Abe R: Soluble Fas ligand: is it a critical mediator of toxic epidermal necrolysis and Stevens-Johnson syndrome? J Invest Dermatol 127:744-745, 2007
8. Nessif A, Moslehi H, Le Gouvello S, Bagot M, Lyonnet L, Michel L, Boumsell L, Bensussan A, Roujeau JC: Evaluation fo the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol 123:850-855, 2004
9. Nuttall TJ, Malham T: Successful intravenous human immunoglobulin treatment of drug-induced Stevens-Johnson syndrome in a dog. J Small Anim Pract 45:357-361, 2004
10. Pereira FA, Mudgil AV, Rosmarin DM: Toxic epidermal necrolysis. J Am Acad Dermatol 56:181-200, 2007
11. Scott DW, Miller WH, Griffin CE: In: Muller and Kirks Small Animal Dermatology, 6th ed., pp. 729-742. Saunders, Philadelphia, PN, 2001
12. Stur K, Karlhofer FM, Stingl G: Soluble FAS ligand: a discriminating feature between drug-induced skin eruptions and viral exanthemas. J Invest Dermatol 127:802-807, 2007
13. Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J, French LE: Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin, Science 282:490-493, 1998