Signalment:
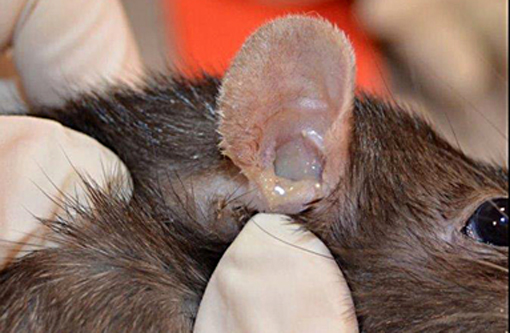
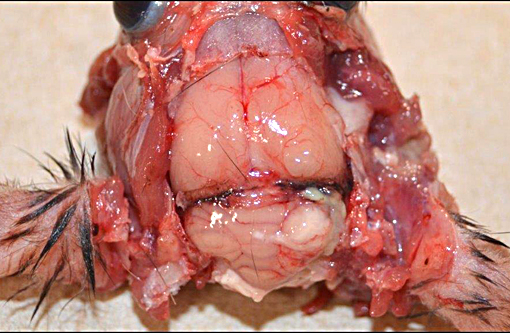
Gross Description:
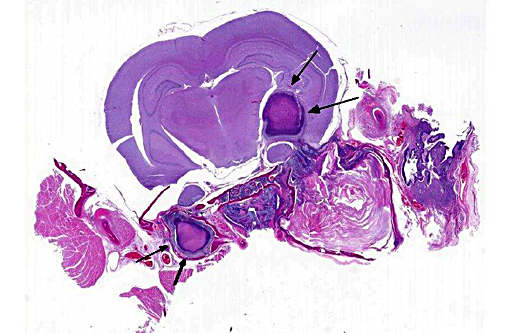
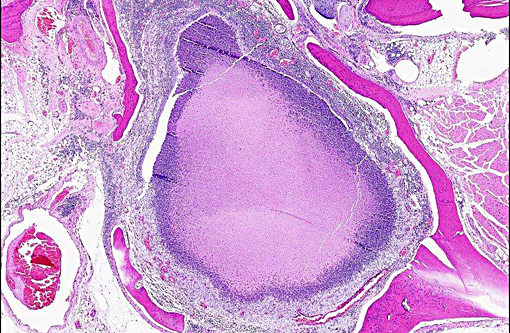
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Primary or secondary bacterial infection is virtually ubiquitous in otitis media, and bacterial ascension through the tympanic bulla or via the Eustachian tube are proven routes of entry. Evidence for hematogenous infection remains circumstantial. The progressive pathology of otitis media is well-described.(15) To reflect the clinical progression of OM, otolaryngology provides an instructive classification scheme: 1) Otitis Media with Effusion (OME) corresponds to asymptomatic persistent middle ear effusion, 2) Acute Otitis Media (AOM) is recurrent middle ear effusion with clinical signs, and 3) Chronic Suppurative Otitis Media (CSOM) refers to discharge through a perforated tympanic membrane for greater than 2 weeks.(11) Complications of OM include hearing impairment, vestibulopathy, extension to otitis interna, and meningoencephalitis. Globally 21,000 people die annually due to OM-related complications.(7) The presence of stratified squamous epithelium, goblet cell metaplasia/hyperplasia and mucus-producing submucosal glands in the middle ear is consistent with a chronic suppurative otitis media in this rat. Although not evident in the current case, concurrent otitis interna was strongly suspected due to extension of the inflammatory process into the brain.Â
Clinical signs of OM are ear pain, odor, head tilt, vestibular signs, scratching at the ear, and neurologic signs related to meningoencephalitis. Discharge/debris from and below the ear canal may be seen. Otoscopic examination may reveal an inflamed tympanic membrane, pus behind the membrane, or membrane rupture.(5) Clinical diagnosis can be aided by radiography, CT scan, cytology, and bacteriology.Â
Organisms commonly implicated in otitis media in rats are Mycoplasma pulmonis, Streptococcus pneumonia, Pasteurella pneumotropica, Staphylococcus sp., Corynebacterium kutscheri, and Klebsiella sp.(5) In this case Mycoplasma culture of the ear, brain, and lung was negative. Prevotella melaninogenica (formerly Bacteroides melaninogenicus) is an anaerobic gram-negative bacillus. Of the anaerobic gram-negative bacilli involved in animal diseases, Fusobacterium is the most important genus, and other genera include Bacteroides, Dichelobacter, and Porphyromonas. P. melaninogenica is also classified as a black pigmented bacterium due to its production of black iron metabolites on blood-containing media; thus, the species name is a misnomer as melanin pigments are tyrosine-based. Virulence factors include hemolysin, neuraminidase, and collagenase.(3,12) P. melaninogenica is a component of the normal flora in the rumen and the human oral cavity, gut and vagina. It is mainly a periodontal pathogen but also considered to be an emerging pathogen in human OM.(1,10) A search of the current veterinary literature identified P. melaninogenica as a common pathogen in bovine footrot and bite wounds from dogs and cats, but no reports of otitis in rats were found.
JPC Diagnosis:
- Brain: Meningoencephalitis, suppurative, focally extensive, with neural degeneration.Â
- Middle ear: Cholesteatoma.
- Middle ear: Otitis media, suppurative, focally extensive, with mucosal ulceration, squamous metaplasia, and bone remodeling.Â
- Eustachian tube: Eustachitis, suppurative, diffuse, chronic-active.Â
- Nasopharynx: Nasopharyngitis, suppurative, diffuse, with mucus metaplasia.Â
- External ear canal: Otitis externa, ulcerative, focally extensive, chronic-active.Â
Conference Comment:
With the common occurrence of ear infections in infants and children, there is an extensive amount of published information regarding their pathogenesis and treatment in people, most of which revolves around the formation of biofilms. Biofilms are complex bacterial communities that adhere to the surface of implanted biomaterial or mucosa and play a major role in chronic ear infections.(6) In addition to enhancing adherence, biofilms increase bacterial virulence by protecting the microbes from immune effector mechanisms and increases their resistance to antimicrobial drugs.(9) In the ear, biofilms may contribute to cholesteatoma formation, as well as suppurative and non-suppurative otitis, and their formation can exacerbate the infection. This is why the first choice of treatment with internal ear infections in people is often surgery, and why all tissue with potential to harbor biofilms must be removed at that time or risk recurrence of infection.(6)
Conference participants were afforded the opportunity to review the intricate and minute anatomy of the outer, middle and inner ear, during which no less than thirty specific anatomical terms were described within the cochlea alone. Nomenclature aside, appropriate function of the ear requires vibration of the tympanic membrane by sound waves, which are then conducted into the cochlea via the ossicles of the middle ear (malleus, incus and stapes) by a push on the oval window. This movement incites a fluid wave within the cochlea, which is completely full of lymph fluid called endolymph. Where along the snail-shaped cochlea this wave intersects with the Organ of Corti and subsequently delivers information to the brain depends on its frequency. Of vital importance to this conduction system is the maintenance of a strong positive endocochlear potential within the endolymph, which requires precise control and recycling of potassium. In fact, potassium recycling defects in the cochlea is an important cause of deafness in people.(2)
References:
1. Brook I. The role of anaerobic bacteria in chronic suppurative otitis media in children: Implications for medical therapy. Anaerobe. 2008;14(6):297-300.
2. Chen J, Zhao HB. The role of an inwardly rectifying K(+) channel (Kir4.1) in the inner ear and hearing loss. Neuroscience. 2014;265:137-146.
3. Eley BM, Cox SW. Proteolytic and hydrolytic enzymes from putative periodontal pathogens: characterization, molecular genetics, effects on host defenses and tissues and detection in gingival crevice fluid. Periodontol. 2000. 2003;31:105-24.
4. Ginn PE, Mansell JE, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier; 2007:677-693.
5. Joint Pathology Center. VSPO S-M02.
6. Kaya E, Dag I, Incesulu A, Gurbuz MK, Acar M, Birdane L. Investigation of the presence of biofilms in chronic suppurative otitis media, nonsuppurative otitis media, and chronic otitis media with cholesteatoma by scanning electron microscopy. Sci World J. 2013:638715. doi: 10.1155/2013/638715. eCollection 2013.
7. Lingen MW. Head and neck. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier Saunders; 2015:740.
8. Maniu A, Harabagiu O, Perde Schrepler M, Catana A, Fanuta B, Mogoanta CA. Molecular biology of cholesteatoma. Rom J Morphol Embryol. 2014;55(1):7-13.
9. McAdam AJ, Milner DA, Sharpe AH. Infectious diseases. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier Saunders; 2015:349.
10. Monasta L, Ronfani L, Marchetti F, et al. Burden of Disease Caused by Otitis Media: Systematic Review and Global Estimates. PLoS One. 2012;7(4):e36226.
11. Morris PS, Leach AJ. Acute and Chronic Otitis Media. Pediatr Clin North Am. 2009 Dec;56(6):1383-99.
12. Soukos NS, Som S, Abernethy AD, et al. Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother. 2005;49(4):1391-6.
13. Verdaguer JM, Trinidad A, Gonz+�-�lez-Garc+�-�a JA, et al. Spontaneous otitis media in Wistar rats: an overlooked pathology in otological research. Lab Anim (NY). 2006;35(10):40-4.
14. Welkoborsky HJ. Current concepts of the pathogenesis of acquired middle ear cholesteatoma. Laryngorhinootologie. 2011;90(1):38-48.Â
15. Wilcock, BP. Eye and ear. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier; 2007:546-551.