Signalment:
7-month-old male
Angus ox (
Bos taurus)A previously
healthy bull calf was presented to the Veterinary Teaching Hospital the day it
was found down and unresponsive.
Gross Description:
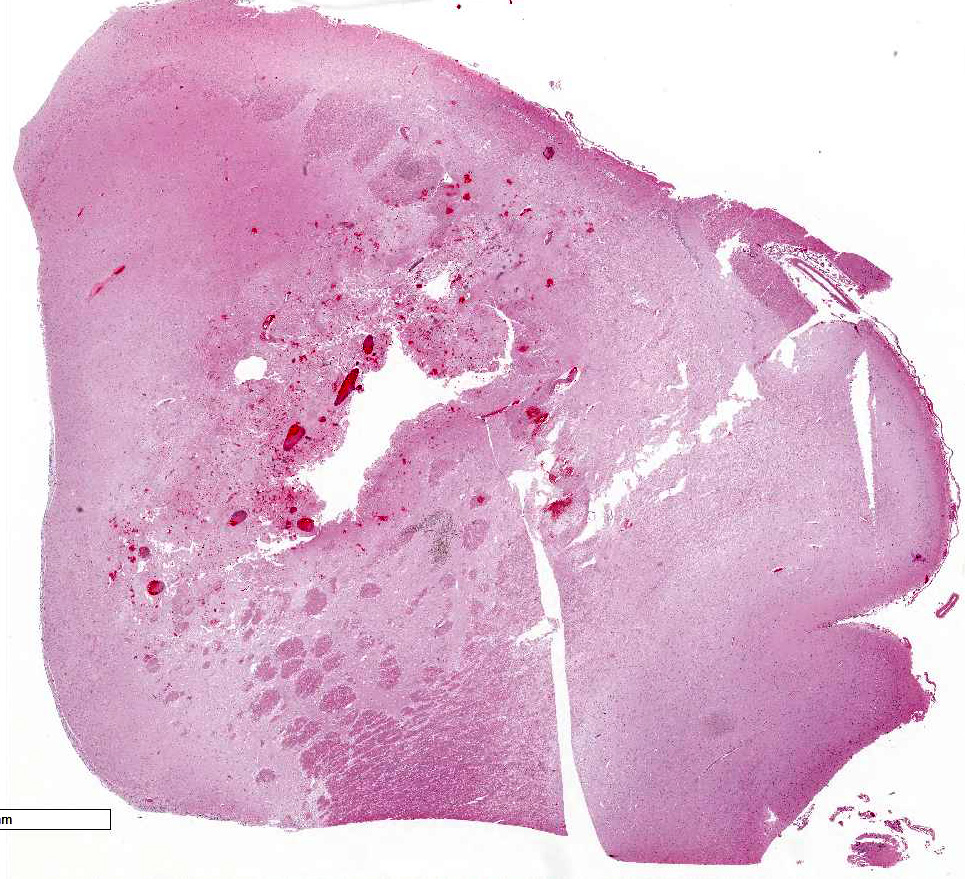
The brain is wet
and soft with mildly dilated ventricles and excessive transparent watery
cerebrospinal fluid. A 1 cm x 1.5 cm dark pink, softened and cavitated focus is
in the left cerebral hemisphere, adjacent to the caudate nucleus and rostral to
the optic chiasm. Less distinct foci of reddening and softening are found elsewhere
in the meninges and parenchyma of the brain and spinal cord.
Mandibular,
parotid, and cervical lymph nodes are enlarged to 2 cm in diameter. Petechiae
and ecchymoses are scattered through many skeletal muscles, esophageal
adventitia, pulmonic trunk adventitia, visceral and parietal pleura, small
intestinal serosa, and urinary bladder. The lungs are reddened. One liter of
transparent yellow watery fluid is in the abdominal cavity. The small
intestine, spiral colon, and descending colon have multifocal mucosal red foci.
Several cestodes, up to 70 cm long, are in the jejunal lumen. Blood-tinged
mucus is in the lumen of the descending colon. Gross
lesions are not observed in the pituitary gland, trigeminal nerves and ganglia,
oral cavity, larynx, trachea, heart, thyroid gland, aorta, stomach, spleen,
liver, gallbladder, pancreas, common bile duct, forestomachs, abomasum, adrenal
glands, kidneys, testes, joints, or bone marrow.
Histopathologic Description:
In
sections of cerebrum (submitted slide), brain stem, and spinal cord, many
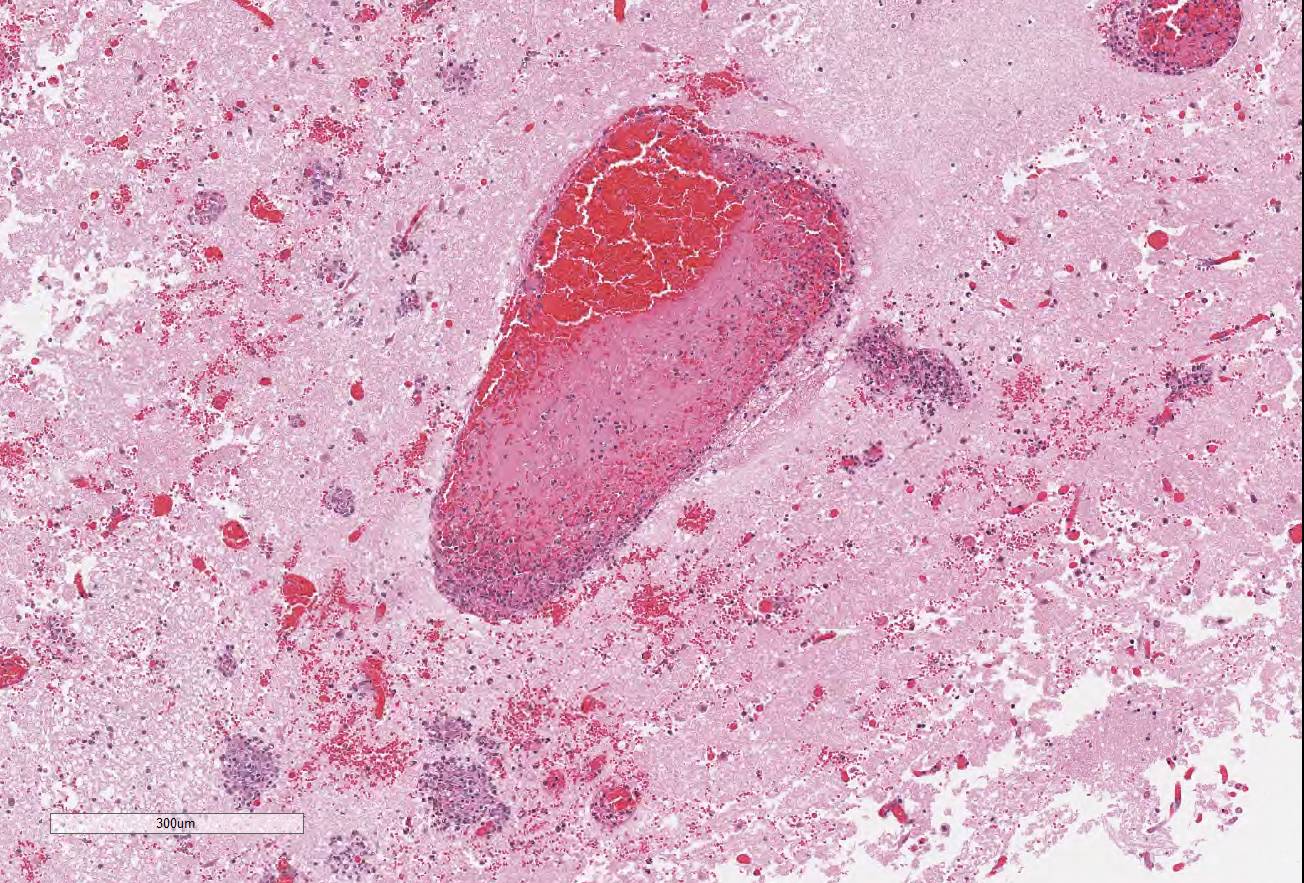
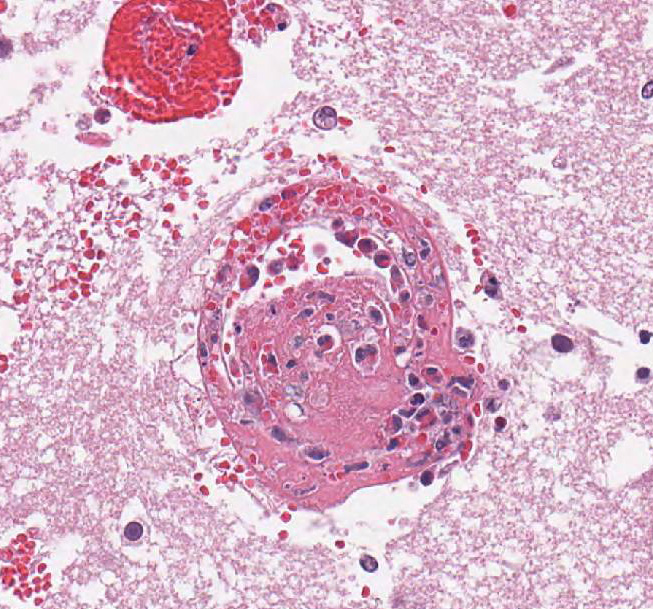
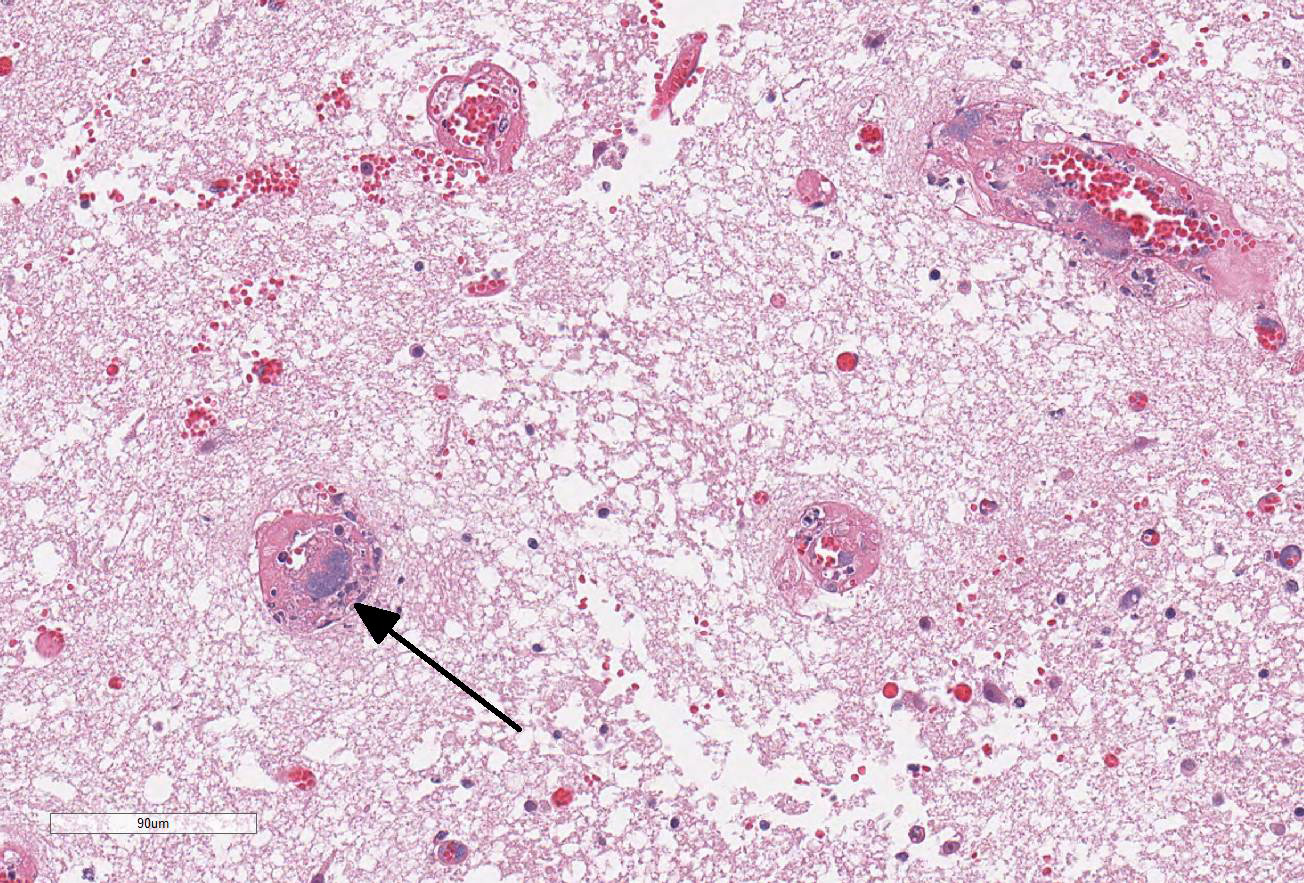
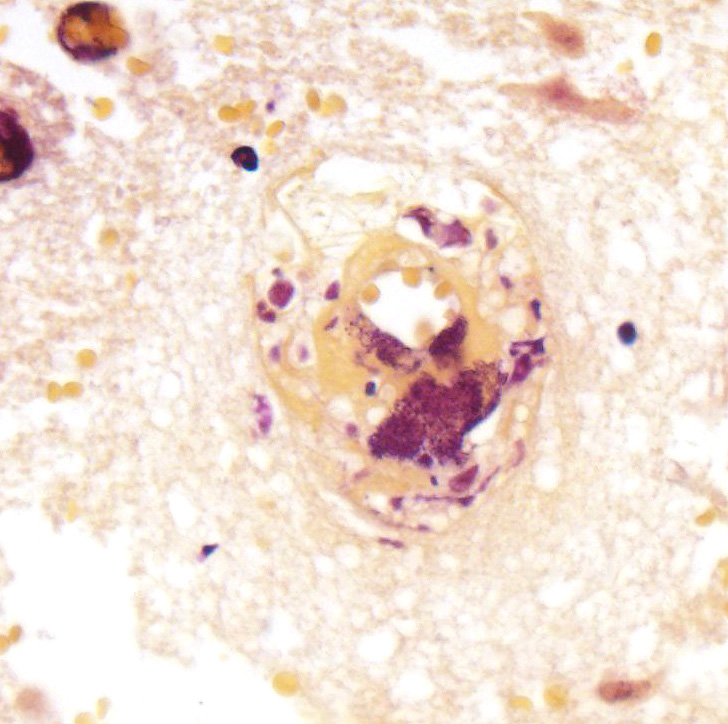
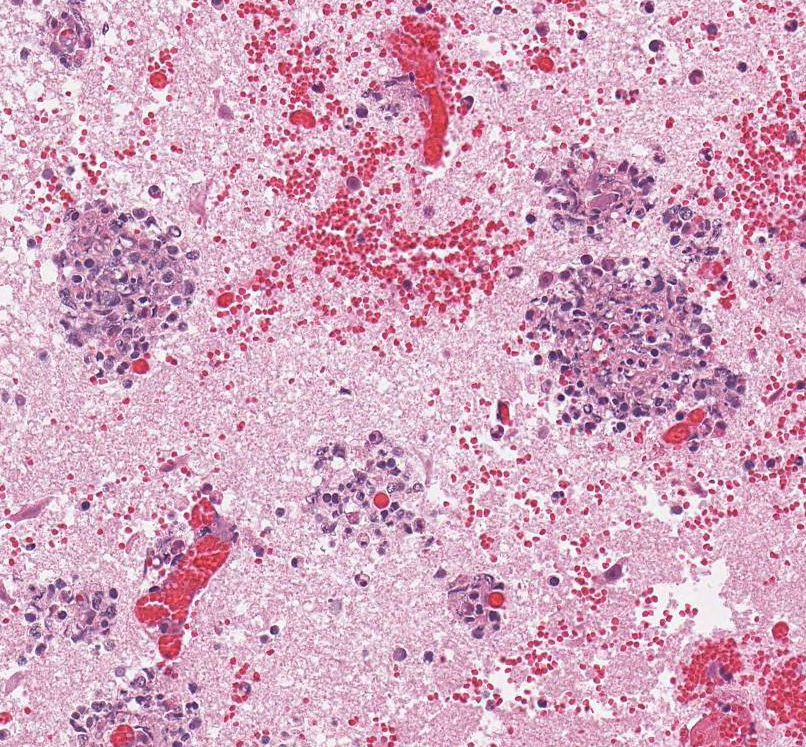
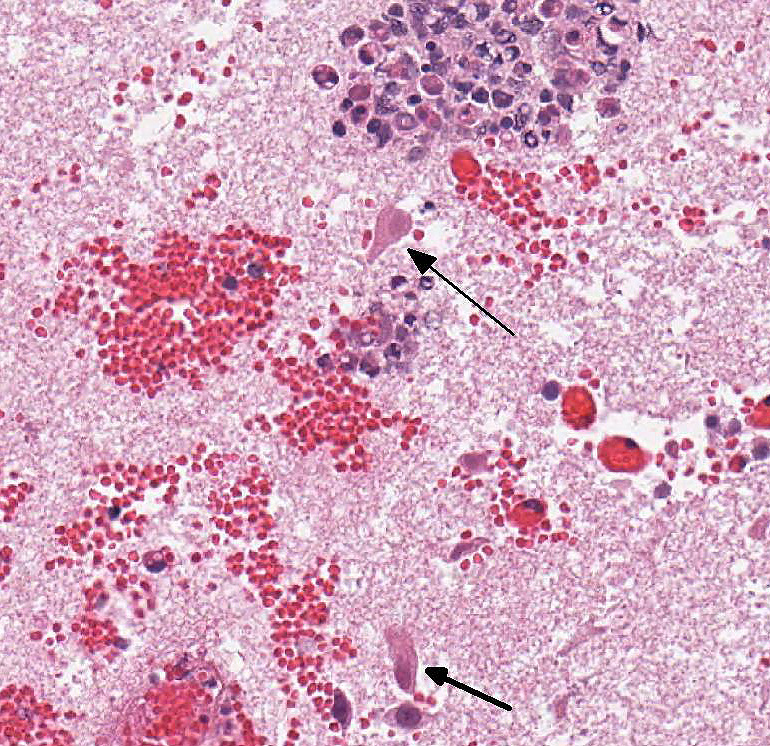
vessels (mainly veins and venules) have poorly organized thrombi rich in
neutrophils. A few venules contain bacteria. The endothelium in affected
vessels is disrupted with transmural extension of neutrophils and fibrinoid
material or frank hemorrhage into Virchow-Robin space and beyond. Thrombi and
hemorrhage are also in the leptomeninges. Surrounding neuroparenchyma is
rarefied with hemorrhage, necrosis, and infiltration by neutrophils with fewer
lymphocytes or macrophages. Thrombi are also in inflamed vessels of the
ventricular myocardium and skeletal muscles. Neutrophils with fewer
lymphocytes and macrophages infiltrate adjacent musculature. Myocytes are
necrotic with hypereosinophilic sarcoplasm and pyknosis or karyorrhexis.
Multifocal hemorrhage, necrosis, and aggregates of neutrophils are in the
tunica media of the pulmonic trunk.
Transmural
hemorrhages in the small intestine are associated with submucosal fibrin
thrombi, pleocellular leukocytic infiltration, and segmental necrosis of
intestinal mucosa. The lung is congested with edematous interlobular septa.
Alveolar spaces contain increased number of macrophages. A few poorly organized
thrombi are in small pulmonary vessels. Evaluated
sections of lymph node, spleen, liver, kidney, adrenal gland, rumen (several
ciliated protozoa), colon, trigeminal nerve and pituitary gland are within
normal limits.
Morphologic Diagnosis:
Thrombotic meningoencephalitis with neutrophilic
vasculitis.
Lab Results:
Aerobic
culture of brain:
Histophilus somni
Bovine
herpesvirus fluorescent antibody test: Negative
Negative virus
isolation
Fluorescent
antibody test for rabies (Indiana State Department of Health): Negative
Fecal
flotation: Numerous trichostrongyle-type eggs as well as eggs/ova of
Moniezia
benedeni,
Nematodirus spp.,
Eimeria spp.
Condition:
Histophilus somni
Contributor Comment:
Rabies had been
included in the clinical differential diagnosis, but the differential diagnosis
at autopsy, based on the presence of multiple malacic hemorrhages in the brain
and spinal cord, included thrombotic meningo-encephalitis and herpesviral
encephalomyelitis. Histologically, the prominent and neutrophilic phlebitis
with bacteria in the lumen of venules, plus the absence of trigeminal
ganglionitis (and a negative FA test for rabies virus), prompted submission of
brain for culture for
Histophilus somni, which was isolated. Lesions of
vasculitis, thrombosis, and inflammation were also detected histologically in
the myocardium, pulmonic trunk, and skeletal muscles, but lung lesions were
minimal in this case. Histophilus
somni is the cause of bovine thrombotic meningoencephalitis (TME), formerly known as
thromboembolic meningoencephalitis (TEME). Currently, vasculitis (mainly
phlebitis) is considered a primary lesion with thrombosis secondary to the
local vasculitis, rather than the result of embolization from a distant site.
2
In fact, the tendency to induce thrombosis is a key feature of
H. somni, and
entails interactions of the bacterium with endothelial cells, leukocytes, and
platelets.
1 The disease is reportedly more common in older calves
and yearlings, and in late fall and early winter;
6 this 7-month-old
calf died on the 23
rd of November.
Lipooligosaccharide
(LOS) is considered the major virulence factor of
H. somni.
1,2
Its diverse activities contribute to the pathogenicity of
H. somni.
Caspase-mediated apoptosis of endothelial cells (and of other host cells)
1
triggered by LOS (probably by its lipid A component), is thought to initiate
the vasculitis of TME.
4 LOS is also thought to play a role in
antigenic mimicry, inflammation (via Toll-like Receptor-4), resistance to
phagocytosis and killing by leukocytes, and evasion of the immune response.
3
Although most
vaccine studies have been focused on the bovine respiratory disease complex,
results suggest a role for humoral immunity. Macrophages that ingest
H. somni are soon killed
by the bacteria, so are unlikely to play a long-term role in dissemination of
infection. This may also suggest that Th1 immunity is less important in
disease control than humoral immunity.
3
JPC Diagnosis:
Brain: Vasculitis, fibrinous and necrotizing, multifocal, severe,
with thrombosis, infarction, and numerous colonies of coccobacilli, Angus,
Bos
taurus.
Conference Comment:
The
contributor provides an outstanding example of the hallmark lesions of
Histophilus
somni in the brain of a feedlot calf. Conference participants localized the
examined tissue section to the corpus striatum of the cerebrum due to the
heterogeneous mix of white and grey matter tracts.
H. somni is a
facultative anaerobic gram-negative coccobacillus that is a normal commensal
bacterium of the bovine genital tract and nasal cavity.
2 In 6 to
12-month-old calves, infection usually occurs following a stressor, such as
transportation, inclement weather, crowding, or changes in diet.
2,3,6
Virulent strains of
H. somni often cause septicemia resulting in a wide
variety of lesions secondary to vasculitis and thrombosis caused by virulence
factor, lipooligo-saccharide (LOS), discussed by the contributor above. Typical
lesions associated with
H. somni include thrombotic meningoencephalitis, myocarditis,
mastitis, metritis, orchitis, conjunctivitis, necrotizing laryngotracheitis,
and polyarthritis H. somni fibrinopurulent broncho-pneumonia as
part of the bovine respiratory disease complex.
2,3,6 Readers are
encouraged to read for a review of the bovine respiratory disease complex. Small
ruminants, bighorn sheep (
Ovis canadensis), and North American bison (
Bison
bison) can also be affected; although the clinical manifestations are often
not as severe likely due to less intensive management practices in these
species.
6
While
the histologic lesions of
H.somi are widespread, the bacteria has a
tropism for the small venules of the cerebral vascular tissue and the most
severe changes often occur within the brain, as in this case.
2
Affected calves often acutely die without treatment. In this case, there is
severe fibrino-necrotic vasculitis and thrombi containing numerous coccobacilli
resulting in a focally extensive infarct of the neuroparenchyma. Numerous
colonies of bacteria are also present in the neuropil. Most conference
participants agreed that the hemorrhage and infarction of the brain is a result
of fibrinonecrotic vasculitis rather than a primary necrosuppurative
meningoencephalitis.
As mentioned by the
contributor,
H. somi secretes an endotoxin (LOS) causing caspase
3-mediated apoptosis of endothelial cells leading to vasculitis and thrombus
formation.
2 Recent studies indicate that
H. somni can
also stimulate endothelial cell tissue-factor (factor 3) activity and disrupt
intercellular junctions enhancing pro-coagulant activity on the endothelial
surface.
1 H. somni and LOS also activate
bovine platelets, which further enhances tissue factor activity on the
endothelial surface, upregulates leukocyte adhesion molecules (P-selectin,
E-selectin, and ICAM-1), and initiates endothelial cell apoptosis via the FasL
(caspases 8 and 9).
2,5 They also induce endothelial cell cytokine
and reactive oxygen species production.
3,4,5 The mechanisms of
H.
somni induced vasculitis and thrombus formation are complex and research is
ongoing to fully elucidate the pathogenesis.
References:
1. Behling-Kelly
E, Rivera-Rivas J, Czuprynski CJ. Interactions of
Histophilus somni with
host cells.
Curr Top Microbiol Immunol. 2016; 396:71-87.
2. Cantile
C, Youssef S. Nervous system. Maxie MG ed. In:
Jubb Kennedy and Palmer's
Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA:
Elsevier Saunders; 2016:364-365.
3. Corbeil
LB. Host immune response to
Histophilus somni.
Curr Top Microbiol
Immunol. 2016; 396:109-129.
4. Inzana
TJ. The many facets of lipooligosaccharide as a virulence factor for
Histophilus
somni.
Curr Top Microbiol Immunol. 2016; 396:131-148.
5. Kuckleburg
CJ, McClenahan DJ, Czuprynski CJ. Platelet activation by
Histophilus somni
and its LOS induces endothelial cell pro-inflammatory responses and platelet
internalization.
Shock. 2008; 29:189-196
6. OToole
D, Sondgeroth KS. Histophilosis as a natural disease.
Curr Top Microbiol
Immunol. 2016;396:15-48.