Signalment:
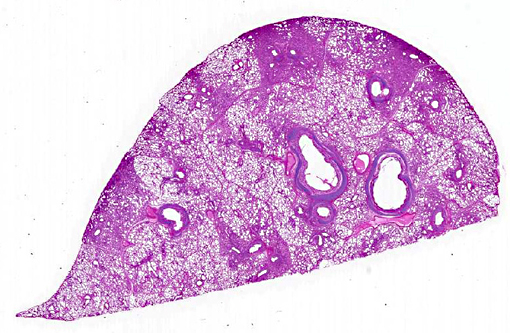
Gross Description:
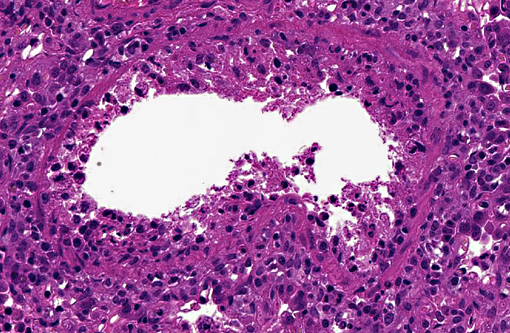
Histopathologic Description:
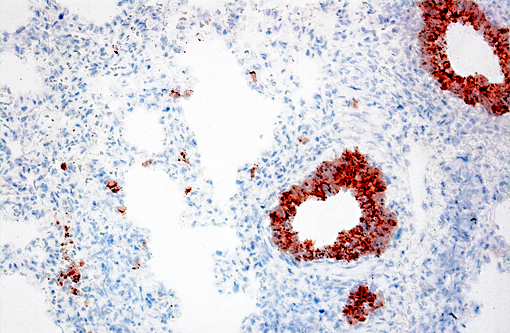
Immunohistochemical analysis to detect type A influenza virus revealed presence of influenza virus matrix antigens in the epithelial cells, with necrotic changes in some bronchioles and adjacent alveolar walls. Other major pathological findings in this case were basophilic intranuclear inclusion bodies (cytomegalic inclusion bodies) in epithelial cells of nasal mucous glands with slight lymphocytic infiltration in the lamina propria and slight inflammatory exudates in the nasal cavity.
Morphologic Diagnosis:
Lab Results:
Virology: Influenza A virus (subtype; H1N2) was isolated from the samples of nasal discharge and lung tissue. No pathologic bacteria were isolated from the lungs.
Immunohistochemistry: Type A influenza virus matrix antigen was detected in the lung. Antigens of porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus type 2 (PCV2), Mycoplasma hyopneumoniae and Mycoplasma hyorhinis were negative in the lung.
Condition:
Contributor Comment:
The common microscopic findings in swine influenza infections are necrotizing bronchitis and bronchiolitis.(3) The influenza virus primarily infects the epithelial cells lining the surface of the respiratory tract, and infection induces cytolysis of infected cells.(9) Cell death induced by the viral infection also occurs through apoptosis induced by the viral components.(7) The term -Ç-ÿbronchointerstitial pneumonia is used in veterinary pathology to describe cases with pulmonary lesions showing histologic features of both bronchopneumonia and interstitial pneumonia.(11) This combined type of pneumonia is frequently seen in many viral infections in which viruses replicate within and cause necrosis of bronchial, bronchiolar, and alveolar cells. The term has also frequently been used in microscopic examination of influenza virus infections in animals.(8) Although histopathologic features of swine influenza are considerably characteristic, immunohistochemical evaluation with sections of formalin-fixed tissue can prove nevertheless useful in differential diagnosis.(10) Similar histological lesions are necrotizing bronchopneumononia induced by PCV2, interstitial pneumonia induced by PRRSV, or mycoplasmal pneumonia.(2,4) Of note, findings on immunohistochemistry were negative for all three diseases in the present case.
JPC Diagnosis:
Conference Comment:
All influenza viruses of significance in swine are type A viruses, with the subtypes H1N1, H1N2 or H3N2 being most common.(6) Influenza is an orthomyxovirus, a single-stranded RNA virus which is well known for its ability to constantly adapt through spontaneous mutations of its hemaglutinin and neuraminidase proteins (antigenic drift) or via recombination of genes with those in strains infecting other species (antigenic shift).(5) It is this talent of recombination which enables cross-species transmission and earns it the distinction of being the deadliest virus known to man, with the 1918 pandemic of H1N1 killing up to 40 million people worldwide.(5)
Early studies demonstrated influenza receptors of both avian and human viruses within the trachea of swine, leading to the labeling of swine as a mixing vessel from which pandemic influenza may arise. It has since been determined that avian influenza can infect humans just as readily as swine, negating the need for the pig as an intermediate host.(6) Thus it is the avian viruses which have been given the most attention as of late, the most noteworthy being the H5N1 strain known as highly pathogenic avian influenza. (see WSC 2012 conf 14, case 4 or WSC 2013 conf 7, case 4 for two examples).
Influenza is capable of cross-infection between swine and people, with the 2009 H1N1 pandemic being the most well-known example. Triple reassortments, which are influenza viruses circulating in swine that possess both avian- and human-origin genes, have been identified which add to the circulating pool likely resulting in the increased incidence of newly reassorted viruses.(6) Newly reassorted viruses pose a significant biosecurity and management problem for the swine industry, with the need for protection against the increasingly antigenically diverse viruses being of significant concern.
In addition to hemagglutinin (used for attachment to and internalization of host cells) and neuraminidase (which prevents viral progeny aggregration), the proteins that influenza is classified by, there are several others important for disease pathogenesis and diagnostics. The polymerase PB2 directs cell processes toward virus replication and is considered most significant of the polymerases with regard to pathogenicity. The nonstructural protein PB1-F2 also contributes to virulence through four mechanisms: inducing apoptosis, IFN suppression, increasing viral replication rates or delaying viral clearance, and increasing inflammation. NS1, another nonstructural protein, interferes with antiviral response and exhibits both proapoptotic and antiapoptotic activities. It also is only expressed in infected cells, making its antibodies a useful naturally occurring DIVA (Differentiating Infected from Vaccinated Animals) tool. The nucleoprotein (NP) is a highly-conserved, internal protein in all type A influenza viruses and thus an appropriate target for ELISA.6 This could potentially alleviate the demonstrated problems associated with antigenic variation when detecting hemagglutinin antibody.
Early detection of different subtypes of influenza virus, most notably H5N1 which experimentally only induces subclinical disease in swine(1), is a significant public health concern. New techniques utilizing oral secretions collected by the suspension of absorbent ropes within the pens of swine may be the way forward for rapid screening in large facilities, though antibody-based diagnostics from oral fluids are not yet a proven technology.(6)
References:
1. Buehler J, Lager K, Vincent A, Miller C, Thacker E, Janke B. Issues encountered in development of enzyme-linked immunosorbent assay for use in detecting Influenza A virus subtype H5N1 exposure in swine. J Vet Diagn Invest. 2014;26(2):277-281.
2. Grau-Roma L, Segales J. Detection of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, swine influenza virus and Aujeszky's disease virus in cases of porcine proliferative and necrotizing pneumonia (PNP) in Spain. Vet Microbiol. 2007;119(2-4):144-151.
3. Haesebrouck F, Pensaert MB. Effect of intratracheal challenge of fattening pigs previously immunised with an inactivated influenza H1N1 vaccine. Vet Microbiol. 1986;11(3):239-249.
4. Hansen MS, Pors SE, Jensen HE, Bille-Hansen V, Bisgaard M, Flachs EM, et al. An investigation of the pathology and pathogens associated with porcine respiratory disease complex in Denmark. J Comp Pathol. 2010;143(2-3):120-131.
5. Husain AN. The lung. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA:Elsevier Saunders; 2015:76.
6. Janke BH. Influenza A virus infections in swine: pathogenesis and diagnosis. Vet Pathol. 2014;51(2):410-426.
7. Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. In vivo induction of apoptosis by influenza virus. J Gen Virol. 1995;76(Pt 11):2869-2873.
8. Prescott JF, Wilcock BP, Carman PS, Hoffman AM. Sporadic, severe bronchointerstitial pneumonia of foals. Can Vet J. 1991:32(7):421-425.
9. Seo SH, Webby R, Webster RG. No apoptotic deaths and different levels of inductions of inflammatory cytokines in alveolar macrophages infected with influenza viruses. Virology. 2004;329(2):270-279.
10. Vincent LL, Janke BH, Paul PS, Halbur PG. A monoclonal-antibody-based immunohistochemical method for the detection of swine influenza virus in formalin-fixed, paraffin-embedded tissues. J Vet Diagn Invest. 1997;9(2):191-195.
11. Lopez A. Respiratory system, mediastinum, and pleurae. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby;2012:458-538.