Signalment:
Gross Description:
Histopathologic Description:
Telecephalon:
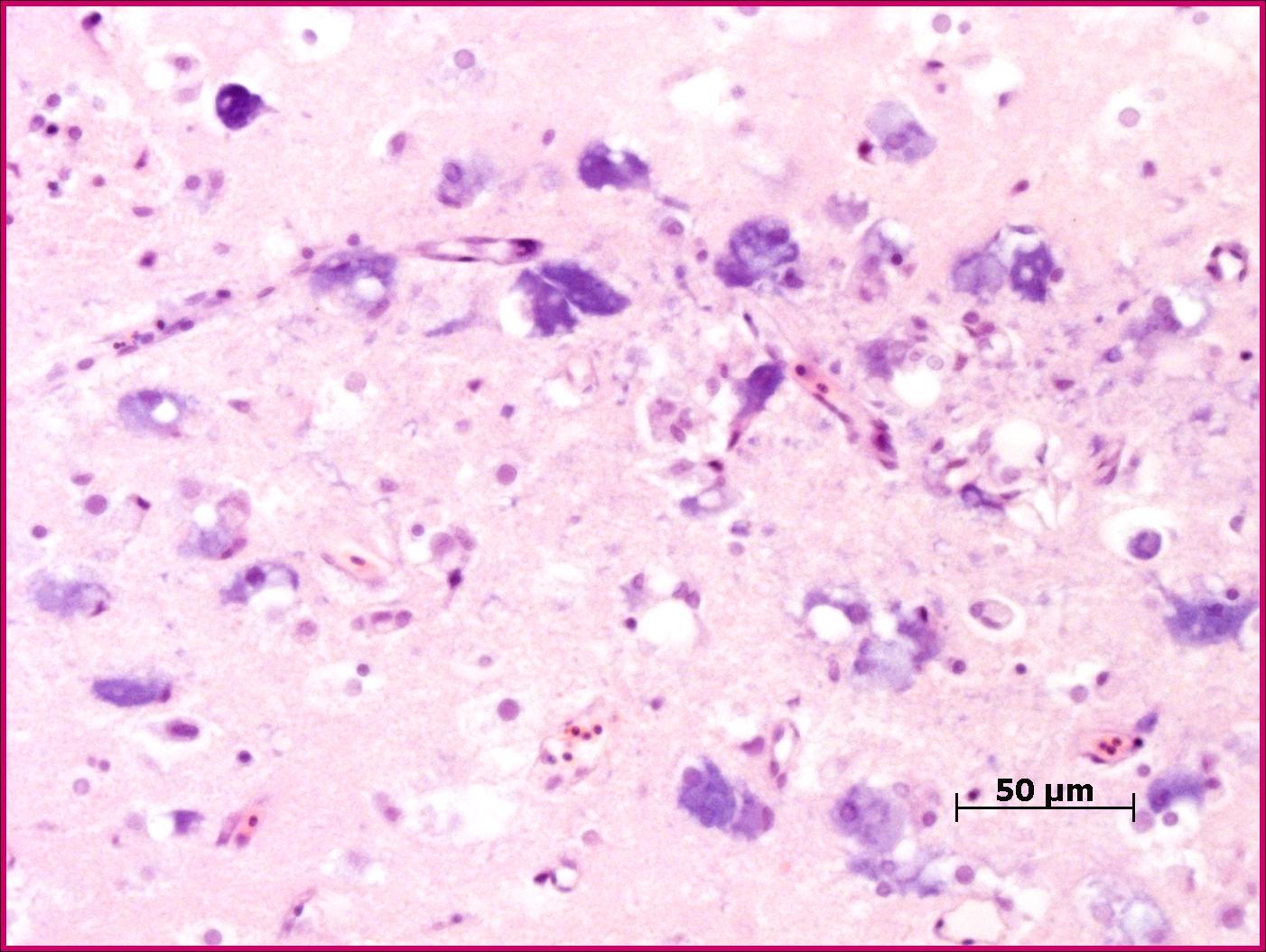
The most prominent changes are present in the olfactory lobe of the telencephalon. There are neurons present with clear cytoplasmic vacuoles, often with scalloped edges (Fig. 2-1). The affected neurons are typically markedly basophilic, and in more advanced cases there is more prominent neuronal degeneration with mild gliosis. Some cases have similar changes in more caudal aspects of the brain, often extending into the cranial spinal cord.
Eye:
The posterior chamber commonly contains a lightly eosinophilic fluid admixed with loose histiocytic cells. There are commonly adherent to the surface of the lens, and occasionally to the retina. Within the retina, sparsely dispersed vacuolated neurons are present predominantly in the inner nuclear layer and nerve fibre layer.
Morphologic Diagnosis:
Lab Results:
Immunohistochemistry Positive for nodavirus
Condition:
Contributor Comment:
Nodaviruses are species specific non-enveloped, icosahedral agents, 25-30nm in diameter.1,5 A large number of marine species are affected by this family of viruses which cause significant mortality in juveniles. The viruses have been a major impediment to the commercialization of numerous fish species. Consistently, the virus affects the central nervous system (with some exceptions noted spinal ganglia of Japanese parrotfish).5 Characteristically the lesions are in the anterior section of the brain (telencephalon in most fish, the olfactory lobe comprises the largest portion) and consist of the presence of vacuoles in the grey matter which appear to be cytoplasmic. Other lesions included pyknosis and basophilia of affected cells. Similar lesions are noted in the neural component of the retina.1,4,5 The relative lack of gliosis is likely due to the acute nature of the infection.
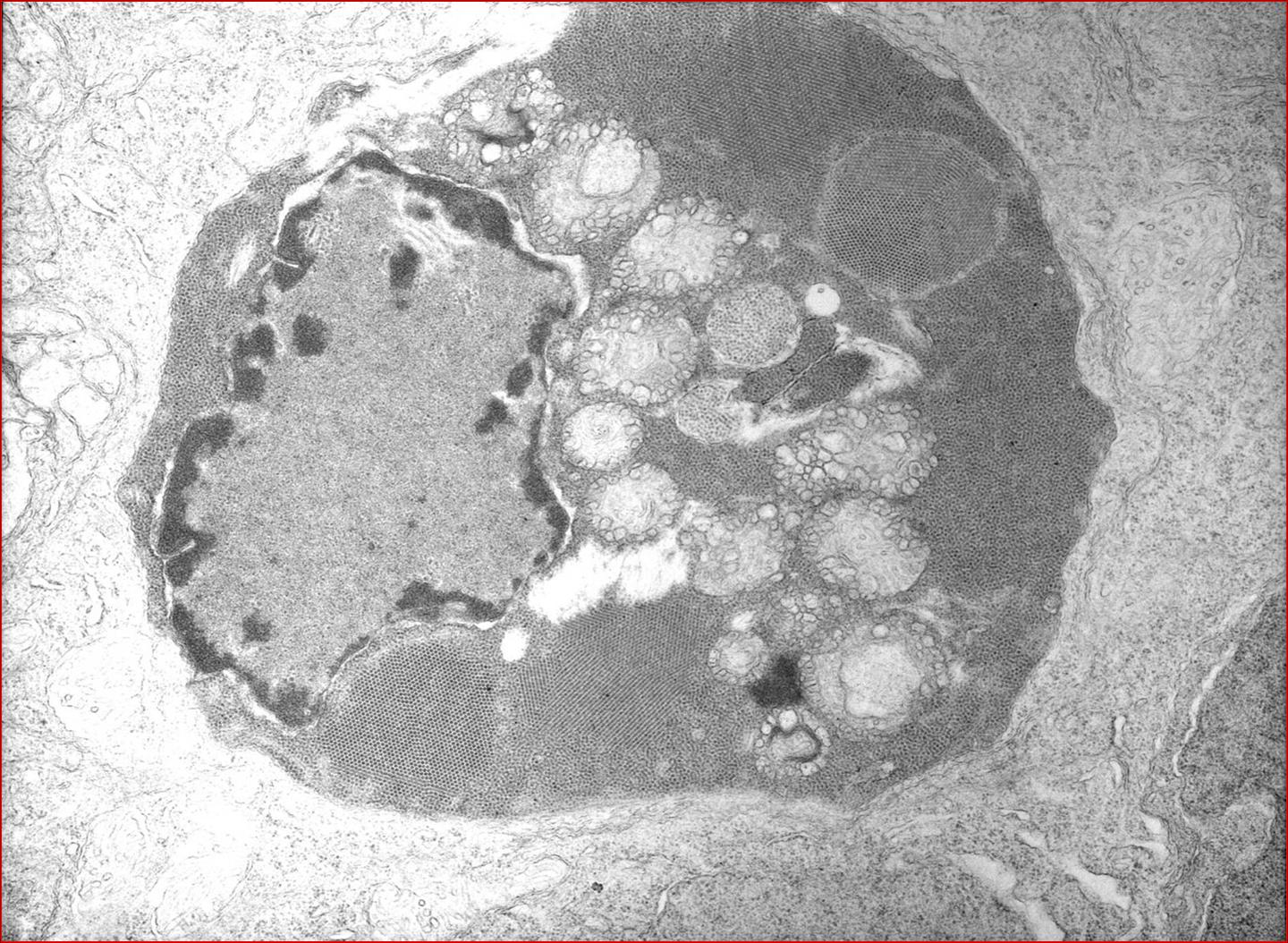
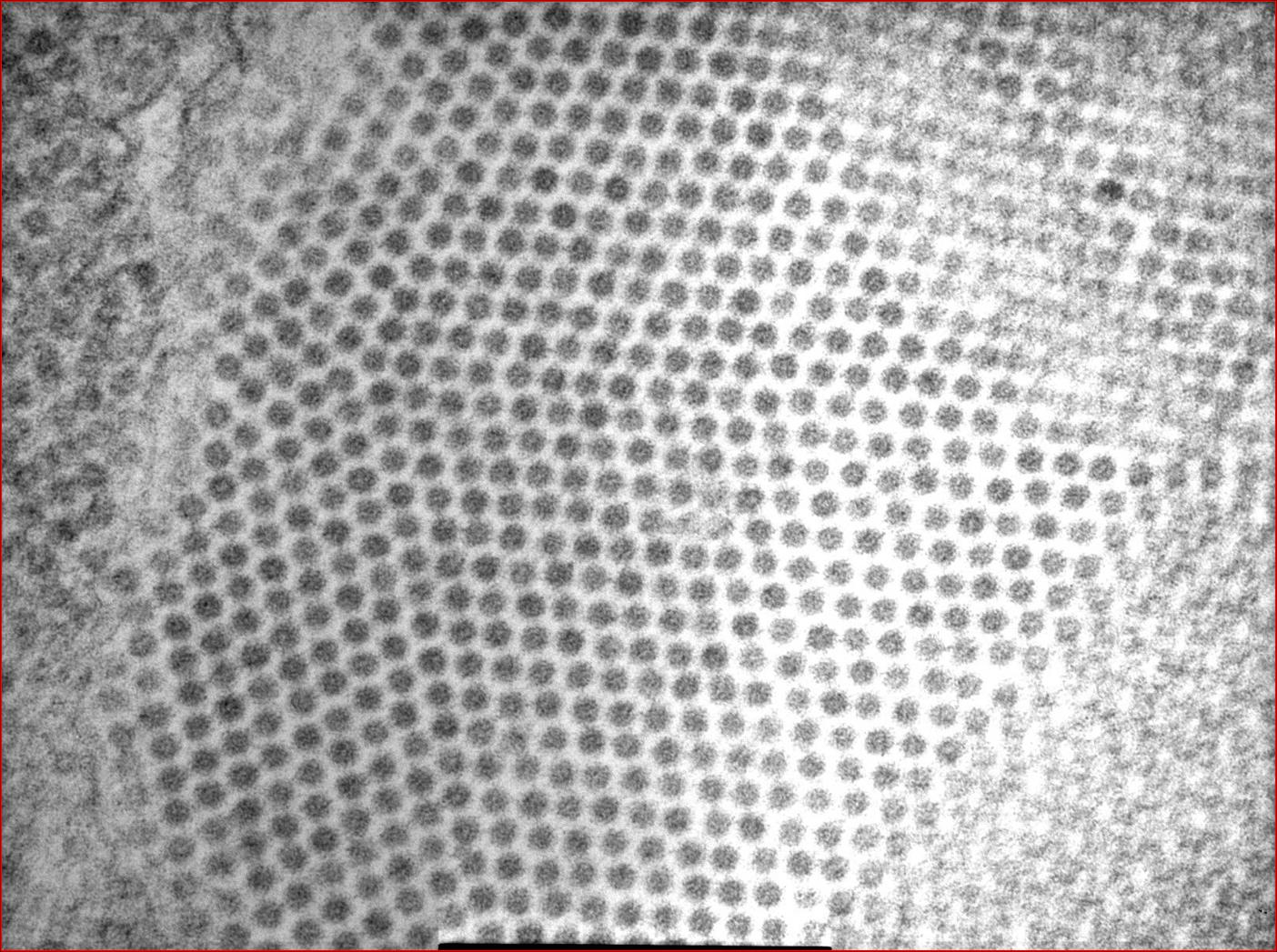
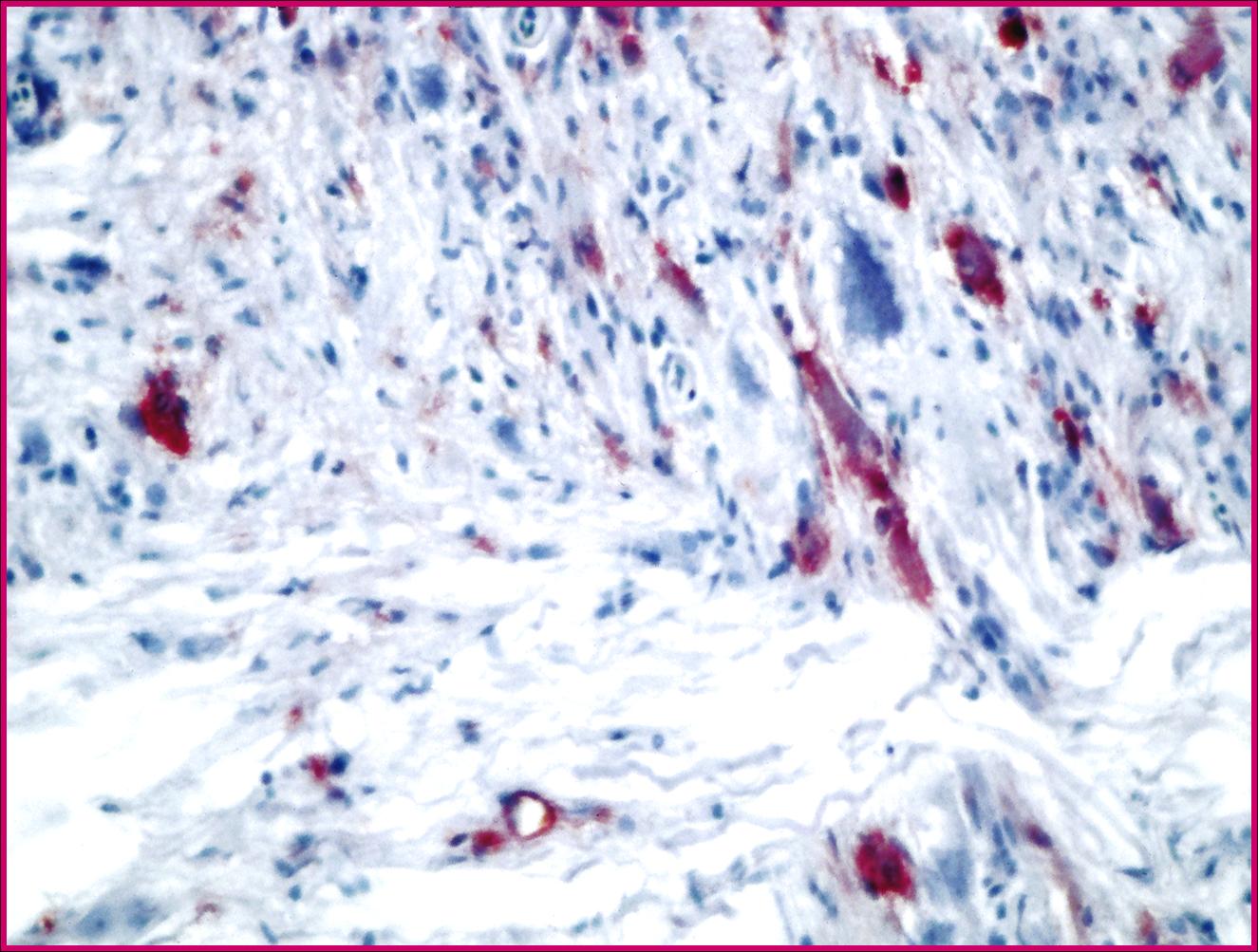
Participants are referred to the electron microscopic images (Figs. 2-2 and 2-3) which display large numbers of intracytoplasmic viral particles. Note the degenerating mitochondria (M) in figure 2-2. An immunohistochemistry image is also submitted demonstrating strongly positive neurons in the telencephalon (Fig. 2-4).
Nodavirus encephalitides are common in the Mediterranean and Indo-Pacific regions, however, recently (2002) a nodavirus of Atlantic cod was demonstrated along the Atlantic seaboard of Canada.3
From a comparative view point, the inflammatory response in teleosts differs somewhat from the higher vertebrates. Whilst teleosts possess oligodendrocytes and astrocytes, it has not been reported that they possess microglia. Additionally, in teleosts even mature ependymal cells retain the capacity for differentiation, implying that neuronal regeneration is possible.3
JPC Diagnosis:
Conference Comment:
Characteristic histologic features of vacuolation and degeneration occur most frequently in the anterior brain. Additional lesions include focal pyknosis and karyorrhexis of neural cells, granularity of neuropil, and mononuclear cell infiltrates.6 There are conflicting reports in the literature on the extent of optic involvement among different species of fish.6 Optical lesions, when described, include vacuolation of the rod and cone layer, as well as ophthalmitis of both the anterior and posterior chambers.6 The cells that most often contain the virus, as identified through electron microscopy, are the neurons, astrocytes, oligodendrocytes, and microglia.6
There is multifocal, moderate histiocytic inflammation of the vitreous body or humor (hyalitis) which was not evident in all sections. Retinal and uveal lesions were not evident in the sections evaluated at AFIP.Â
References:
2. Barker DE, MacKinnon AM, Boston L, Burt MD, Cone DK, Speare DJ, Griffiths S, Cook M, Ritchie R, Oliver G: First report of piscine nodavirus infecting wild winter flounder Pleuronectes americanus in Passamaquoddy Bay, New Brunswick, Canada. Dis Aquat Org 49:99-105, 2002
3. Ferguson HW: Systemic Pathology of Fish, pp. 158-167. Iowa State University Press, Ames, IA, 1989
4. Johnson SC, Sperker SA, Leggiadro CT, Groman DB, Griffiths SG, Ritchie RJ, Cook MD, Cusack RR: Identification and characterization of a piscine neuropathy and nodavirus from juvenile Atlantic cod from the Atlantic coast of North America. J Aquat Anim Health 14:124-133, 2002
5. Munday BL, Nakai T: Special topic review: Nodaviruses as pathogens in larval and juvenile marine fish. World J Microbiol Biotechnol 13:375-381, 1997
6. Munday BL, Kwang J, Moody N: Betanodavirus infections of teleost fish: a review. J Fish Dis 25: 127-142, 2002
7. Samuelsen OB, Nerland AH, J+�-+rgensen T, Schr+�-+der MB, Sv+�-�sand T, Bergh +�-�: Viral and bacterial diseases of Atlantic cod Gadus morhua, their prophylaxis and treatment: a review. Dis Aquat Org 71:239-254, 2006
8. Starkey WG, Ireland JH, Muir KF, Shinn AP, Richards RH, Ferguson HW: Isolation of nodavirus from Scottish farmed halibut, Hippoglossus hippoglossus (L). J Fish Dis 23:419-422, 2000