Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 1
August 22nd, 2018
CASE I: RUSVM-1 (JPC 4020132).
Signalment: 4-year-old, male, African green monkey (Chlorocebus aethiops sabaeus)
History: This monkey was euthanized during a recent outbreak of acutely fatal enteric disease in a colony of captive African green monkeys (Chlorocebus aethiops sabaeus) in the island of St. Kitts, West Indies. On clinical examination, the monkey had bloody diarrhea, was pyrexic and severely dehydrated. Previous to this case submission, multiple monkeys in the same enclosure had died after a short period of illness characterized by depression, diarrhea and dehydration, or had been found dead in the enclosure. Necropsies performed by the referring veterinarian revealed multifocal, variably-sized, white foci throughout the splenic and hepatic parenchyma. All affected monkeys were part of a large, breeding population maintained by the Behavioral Sciences Foundation, Estridge Estate, St. Kitts, West Indies. Maintenance, testing and all procedures carried out in this facility are approved by the Animal Care Committee of the Behavioral Sciences Foundation, acting under the auspices of the Canadian Council on Animal Care.
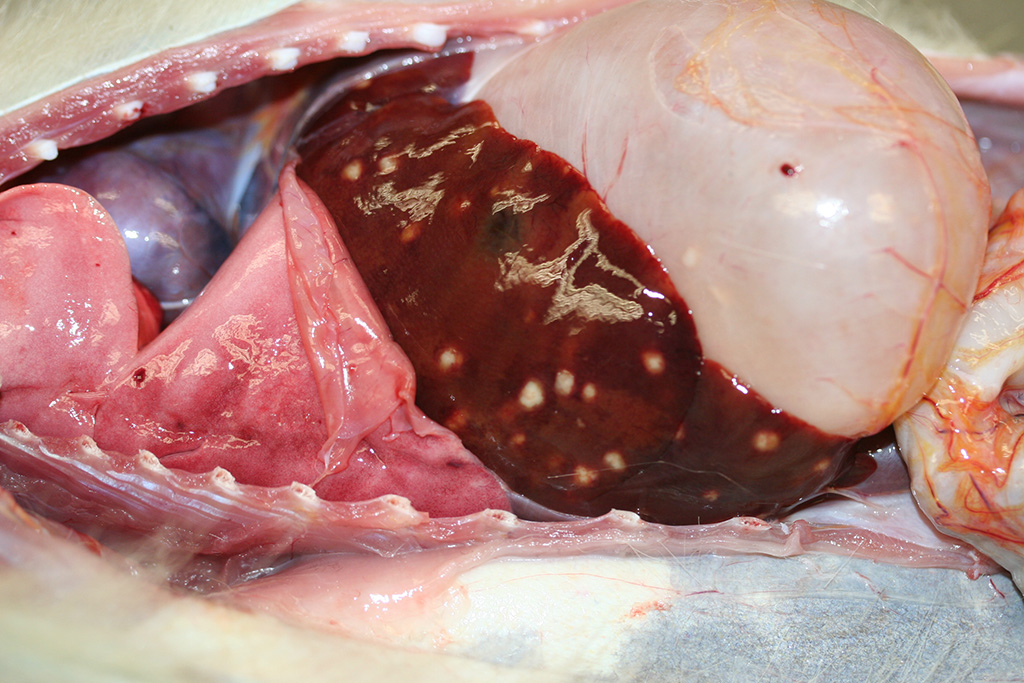
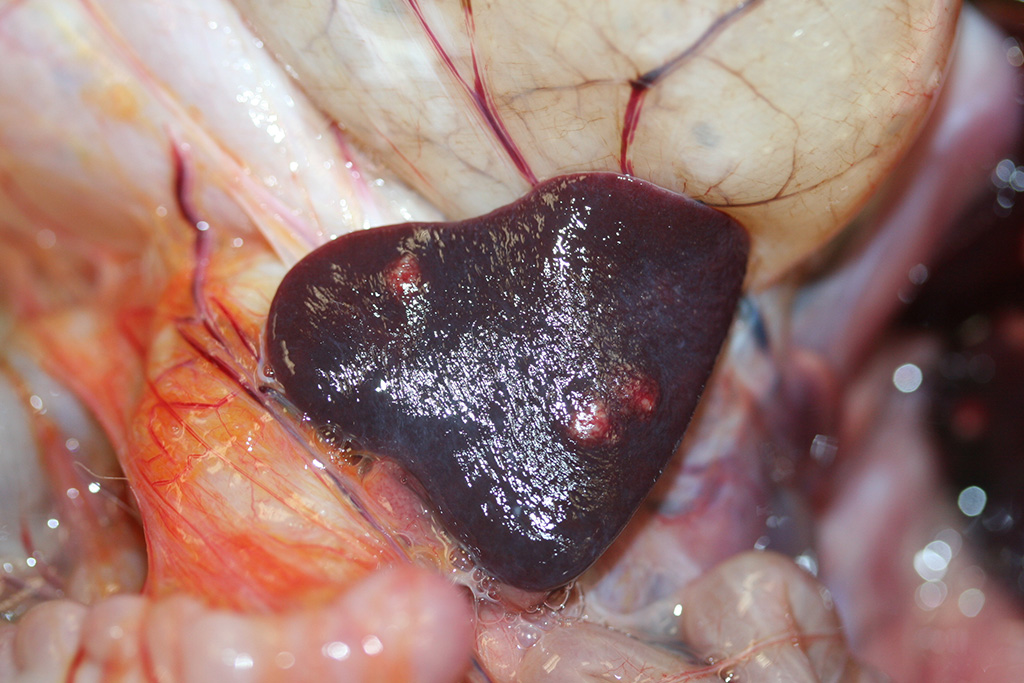
Gross Pathology: At necropsy, the monkey was in poor body condition (BCS 2/5), with scant fat reserves and muscle mass. The carcass was moderately dehydrated and the perineum was stained with blood-tinged feces. Multifocal areas of petechiation were present throughout the subcutaneous tissue, and mucous membranes were diffusely pale. The liver and the spleen had multifocal, variably-sized (2 mm-8mm) white foci. The spleen was slightly enlarged. On cut surface, the foci were moderately firm and had a caseated appearance (abscessation /necrosis). The stomach was markedly distended with gas. The mucosa of the small intestine was diffusely reddened. The cecum and the colon contained blood-tinged mucus and there were numerous 1-2 cm white, slender nematodes present (Trichuris sp.). The mesenteric lymph nodes were moderately enlarged and slightly edematous. No other gross lesions were present elsewhere.
Laboratory results: Bacteria recovered from hepatic and splenic swabs collected during necropsy examination were identified by routine culture and biochemical methods as gram-negative, cytochrome oxidase-negative rods. MicroID kits identified the isolates as Yersinia spp. Further molecular diagnosis provided by amplification and sequencing of the 16S SSU rRNA gene confirmed the isolates as Y. enterocolitica. Leukograms of affected monkeys indicated a leukocytosis most commonly composed of monocytosis, basophilia, lymphocytosis (combined increase in lymphocytes and large granular lymphocytes), an occasional mature neutrophilia, and rarely a left shift. Monocytes were commonly vacuolated. Lymphocytes commonly had increased amounts of pale blue glassy cytoplasm, an occasional reniform nucleus, and more open chromatin. Large granular lymphocytes were commonly larger than a neutrophil with variable numbers of granules and reniform to amoeboid nuclei. Neutrophils commonly had open chromatin and were pale staining. Döhle bodies, cytoplasmic basophilia, and vacuolation (toxic changes) were not common. Many ruptured cells (“basket cells”) were observed. Increased PCV was uncommon, and red cell abnormalities included schistocytes and microcytes; plasma was often pink-tinged. Platelet clumping was common. The biochemical profile occasionally indicated cholestasis, less commonly, hepatocellular damage, and infrequent increases in UN and phosphorus.
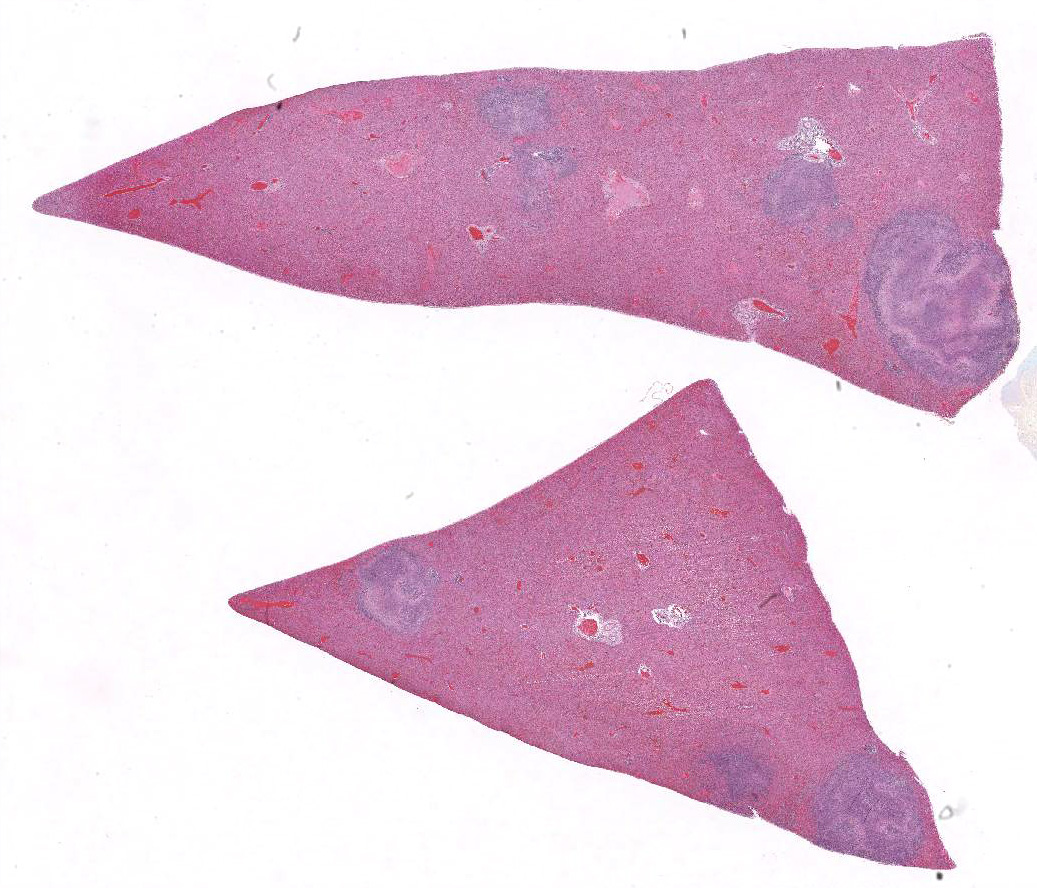
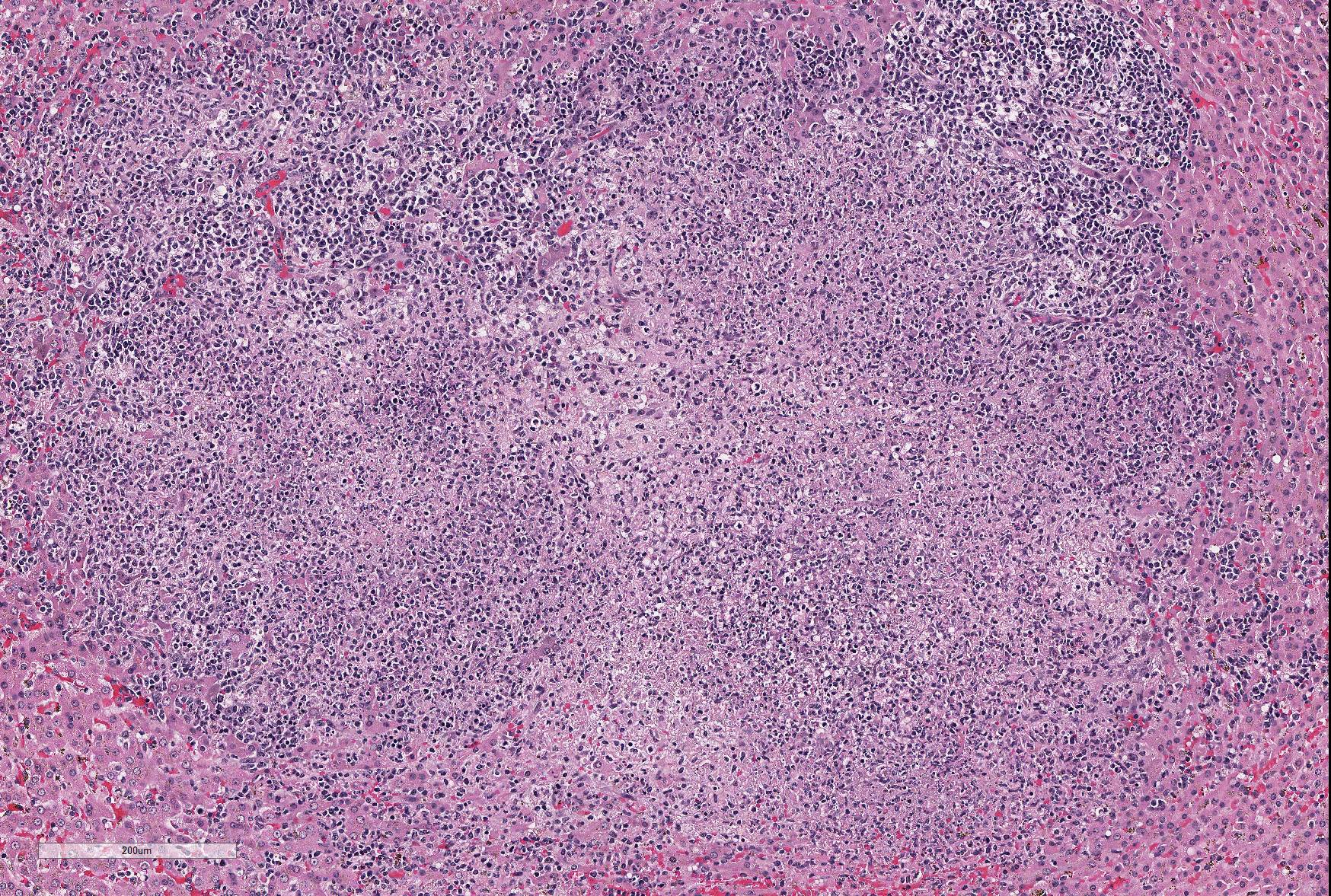
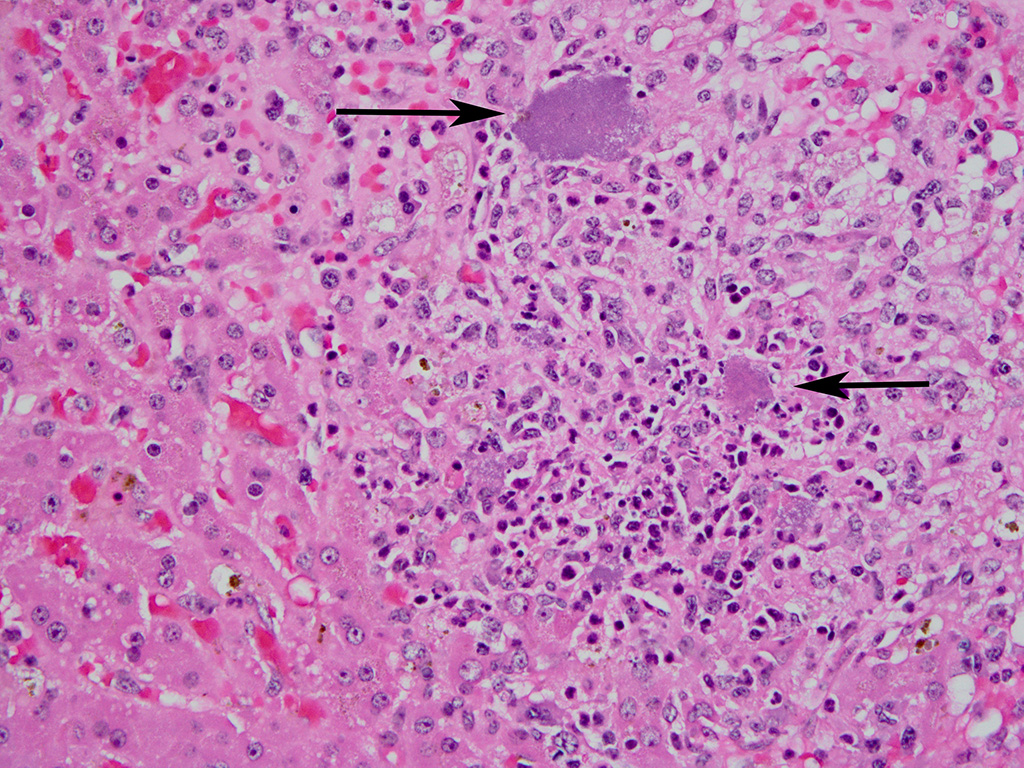
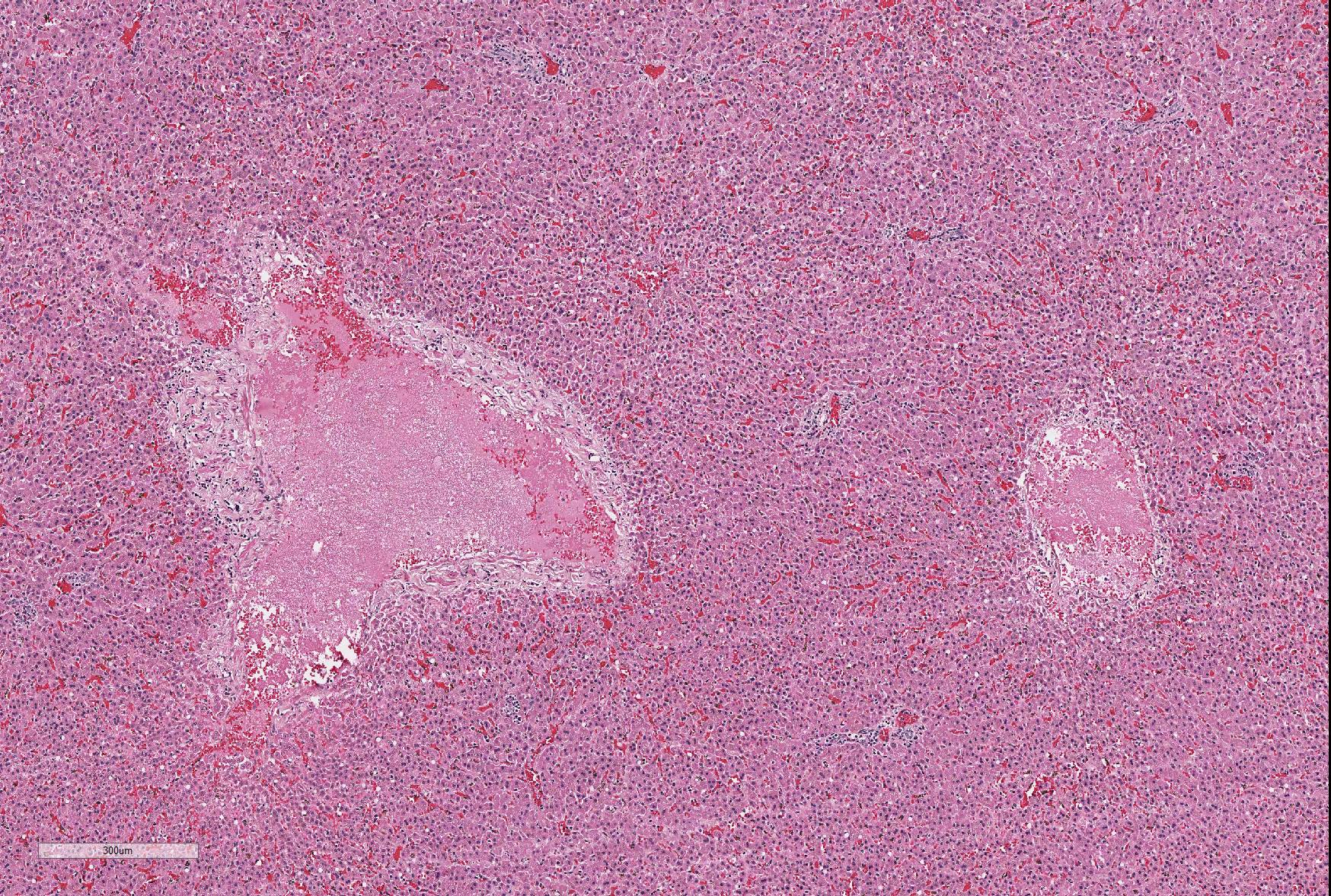
Microscopic Description: Sections of the liver and the spleen reveal random, multifocal to coalescing areas of liquefactive necrosis characterized by extensive loss of normal tissue architecture and cellular detail. Areas of necrosis consisted of a central core of pale eosinophilic and karyorrhectic cellular debris, degenerate neutrophils and a pale, eosinophilic, fibrillar material (fibrin) associated with variably-sized gram-negative colonies (up to 200μm in diameter) of coccobacilli. Numerous macrophages, and lesser numbers of lymphocytes and plasma cells surrounded the areas of necrosis. Additional microscopic lesions present in the liver include disorganization of hepatic cords, centrilobular and sinusoidal congestion and a mild periportal lymphocytic and plasmacytic hepatitis. Numerous hepatocytes and Kupffer cells contained a yellow-brown granular pigment in the cytoplasm and small bile casts were occasionally observed in canaliculi. Additional microscopic lesions present in the spleen included follicular lymphoid hyperplasia and splenic histiocytosis (please note that not all slides contain a section of the spleen). Similar microscopic lesions were present in the mesenteric lymph nodes and the small intestine (not submitted).
Extramedullary hematopoiesis is present.
Contributor’s Morphologic Diagnoses: Liver and spleen: Necrotizing and suppurative hepatosplenitis, multifocal, subacute, with gram-negative bacterial colonies, African green monkey.
Contributor’s Comment: Yersinia enterocolitica is a member of the genus Yersinia, a facultative anaerobic bacterium from the Enterobacteriaceae family. Yersinia enterocolitica is associated with diverse clinical disease manifestations in humans and a variety of animal species, including non-human primates.6,7
Yersiniosis is an important condition in non-human primates. Non-human primates are very sensitive to pathogenic Yersinia, and yersiniosis has been described in these species as an acutely fatal enteric disease, or as a chronic, debilitating, primarily enteric disease characterized by anorexia, diarrhea and weight loss.1,3-5 Enteric yersiniosis in non-human primates is caused by Y. pseudotuberculosis and Y. enterocolitica. Yersinia pestis, the causative organism of plague, is thought to have evolved from Y. pseudotuberculosis and initially was considered an enteric pathogen.6 Numerous reports have been published describing outbreaks of acute fatal yersiniosis with high morbidity and mortality in captive colonies of non-human primates.1,3,5 Host susceptibility to Yersinia may differ depending on monkey species.6 Outbreaks are characterized by variable degrees of weight loss, anorexia, diarrhea and dehydration. Clinical disease manifestation is variable, and highly dependent on bacterial strains.5,6
Yersinia spp. are widespread in the environment. Fecal contamination of water and the environment, as well as food contamination, are commonly recognized sources of infection.1,3 Pigs, wild rodents, and wild birds are major reservoirs of pathogenic Yersinia spp. From the point of view of public and animal health, additional sanitary precautions and extreme caution are necessary in order to avoid intra and interspecies transmission of pathogenic Yersinia spp.
The source of infection in this outbreak has yet to be established, however, wild birds and rodents were commonly observed around the enclosures. It has also been postulated that stress and behavioral factors may precipitate the presentation of severe clinical disease in captive populations of non-human primates since prevalence of infection appears to be high.1,4 Age may also be an important factor in clinical disease manifestation, with asymptomatic infections being common in adults.5 In the outbreak described, the majority of mortalities occurred in juveniles; however as the outbreak progressed, adult monkeys were also clinically affected.
Characteristic postmortem findings reported in non-human primates include severe enterocolitis, necrotic foci in the liver and the spleen and mesenteric lymphadenitis.3-5 Microscopic findings are characterized by the presence of multiple necrotic foci of variable size, containing cellular debris and large gram-negative colonies of coccobacilli. Differential diagnoses for bacteria that appear microscopically as large colonies include Yersinia spp., Actinomyces spp., Actinobacillus spp., Corynebacterium spp., Staphylococcus spp. and Streptococcus spp. (resulting in the mnemonic YAACS).
In this case, the clinical signs, the gross findings and the characteristic microscopic lesions were consistent with Yersinia spp. infection. Amplification and sequencing of the 16S SSU rRNA gene confirmed the isolates as Y. enterocolitica. Antimicrobial susceptibility testing of bacterial isolates obtained indicated tetracycline, gentamycin, chloramphenicol, amikacin, imipenem, ceftiofur, kanamycin, trimethoprim/sulfamethoxazole, ceftriaxone, ciprofloxacin, ceftazidime, pip/tazo con 4, aztreonam, levofloxacin, and cefepime susceptibility. Isolates were resistant to sulfisoxazole, amoxicillin/clavulanic acid 2:1 ratio, ampicillin, amoxicillin, erythromycin, vancomycin, and clindamycin. Outbreak mortalities were significantly reduced after tetracycline administration.
JPC Diagnosis: 1. Liver: Hepatitis, necrotizing, multifocal to coalescing, marked, with large colonies of bacilli.
- Spleen: Splenitis, necrotizing, multifocal, marked with large colonies of bacilli. (spleen not present on all slides.
Conference Comment:
The genus Yersinia is a member of the family Enterobacteriaceae and consists of 14 species and gram-negative bacilli, three of which, Y. enterocolitica, Y. pseudotuberculosis, and the causative agent of plague, Yersinia pestis are pathogenic for humans and nonhuman primates. An additional species, Y. ruckeri is a pathogen of fish. Nonhuman primates and man are considered extremely susceptible to infection by these pathogens, although Y. enterocolitica was not considered as a human or veterinary pathogen until the late 1960s.6 In contrast, a number of domestic and wildlife species including pigs, rodents, and wild birds, are and important reservoirs of these pathogens and considered significantly less susceptible to their pathogenic effects. (Interestingly, pork chitterlings (intestine) have been often identified as a source of food-borne illness to young children, during the cleaning and preparation phase.) Y. enterocolitica has over 60 serotypes, but less than ten are pathogenic in management and nonhuman primates. Stress and behavioral factors may also precipitate the conversion of asymptomatic carriage of pathogenic Yersinia serotypes to active infection; one case report describing an outbreak of fatal yersiniosis was attributed to the administration of metronidazole to treat colony trichomonad infections.1
It is difficult, if not impossible, to distinguish the lesions caused by Y. enterocolitica from those caused by Y. pseudotuberculosis either grossly or histologically, and advanced diagnostics (as done in this case) are recommended in all cases where definitive speciation is required. One study5 suggested that the pathogenicity of Y. enterocolitica is lower than that of Y. pseudotuberculosis, as sudden death may occur in the absence of clinical signs with the latter. This study postulates that the ypm gene carried by Y. pseudotuberculosis (but not Y. enterocolitica) may encode a superantigenic toxin resulting in enhanced pathogenicity.
An interesting historical fact about Y. enterocolitica serogroup 0:8 (a particularly pathogenic serogroup in both man and non-human primates) is the story of some of its earliest outbreaks. In September 1976, two hundred school children in Holland Patent, NY, developed nausea, vomiting, diarrhea, and abdominal cramping. Thirty-six children were hospitalized, sixteen of whom subsequently had an appendectomy. Contaminated chocolate milk was identified as the common source in this outbreak.8 A second major outbreak occurred in a coed summer camp in Liberty, NY, five years later. At least 35% of 455 campers and staff members had similar GI signs as the previous outbreak, with 53% complaining of abdominal pain. Appendectomies were performed on five of the seven campers who were hospitalized.8 This likely reflects the affinity of “hot” gram-negative bacilli for lymphoid tissue, and the ability of this particular bacterium to mimic a surgical emergency.
Conference participants discussed various aspects of the pathogenesis of yersinial invasion into the GI tract and its affinity for M cells (similar to other “hot” gram-negatives), as well as similarities and differences of Y. pseudotuberculosis and enterocolitica as compared to Y. pestis (which has an enteric form with lesions quite similar to those seen in this case.) Additionallly, another ruleout for abdominal infection in African green monkeys, hypermucoviscous Klebsiella pnemoniae (WSC 2015, Conference 1, Case 2) was briefly discussed.
Contributing Institution:
Department of Pathobiology
Ross University School of Veterinary Medicine, St. Kitts, West Indies
References:
- Bakker J, Kondova I, de Groot CW Remarqe EJ, Heidt, PJ. A report on Yersinia-related mortality in a colony of new world monkeys. Lab Prim Newsletter. 2007; 46(3):11-15.
- Bronson R, May B, Ruebner. An outbreak of infection with Yersinia pseudotuberculosis in nonhuman primates. Am. J. Pathol. 1972; 69(2):289-308.
- Iwata T, Hayashidani H. Epidemiological findings on yersiniosis in nonhuman primates in zoological gardens in Japan. Japan Agricultural Research Quarterly. 2011; 45(1):83-90.
- MacArthur JA, Wood M. Yersiniosis in a breeding unit of Macaca fascicularis (cynomologus monkeys). Laboratory Animals.1983; 17:51-155.
- Nakamura S, Hayashidani H, Iwata T, Namae S., Une Y. Pathological changes in captive monkeys with spontaneous yersiniosis due to infection by Yersinia enterocolitica serovar O8. J. Comp Path 2010; 143(2–3):150-156.
6. Sabina Y, Rahman A, Ray RC, Montet, D. Yersinia enterocolitica: Mode of transmission, molecular insights of virulence and pathogenesis of infection. J Path 2011; 1:1-10. - Shayegani M, Stone WB, Deforge I, Deforge I, Root T, Parsons LM, Maupin P. Yersinia enterocolitica and related species isolated from wildlife in New York State. Appl Environ Microbiol 1986; 52: 420-424.
- Sheyegani M, Morse D, DeForge I, Root T, Parsons, LM, Maupin PS. Microbiology of a major foodborne outbreak of gastroenteritis caused by Yersinia enterocolitica serogroup 0:8. J Clin Mico 1983; 35-40.