WSC 22-23
Conference 3
CASE IV:
Signalment:
1-year-old female Wyandotte chicken (Gallus gallus)
History:
This chicken was lethargic, diarrheic and was losing weight. She was found laterally recumbent with her right leg extended behind her and the left leg curled underneath her. Coelomocentesis revealed yellow coelomic fluid. Euthanasia was elected, and the carcass was received for examination. This chicken was part of a small backyard flock of 16 chickens.
Gross Pathology:
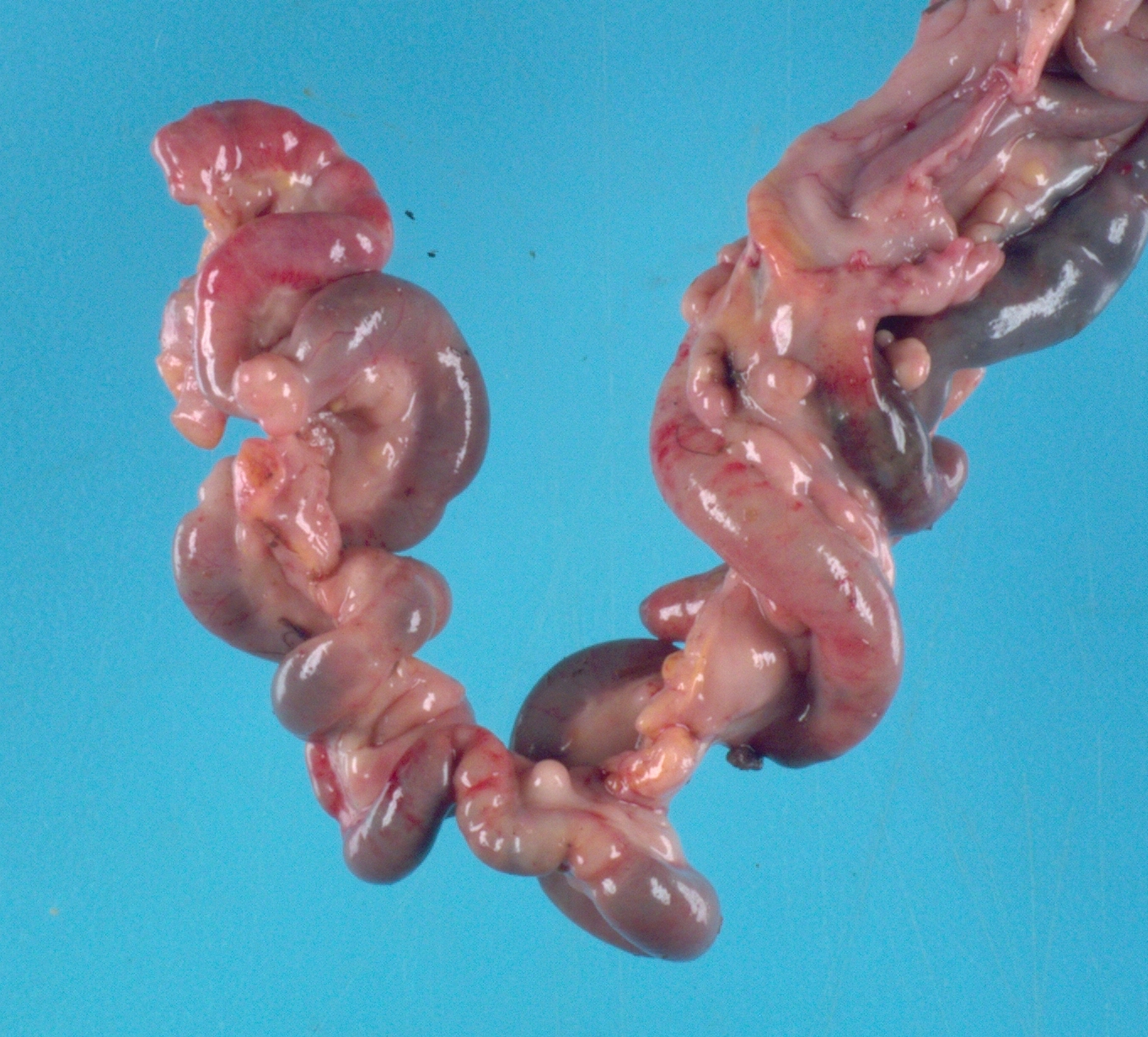
The chicken was in poor body condition with scant visceral fat and moderate pectoral muscle atrophy. There was approximately 100 mL of yellow, turbid, watery fluid within the coelomic cavity. Multifocally throughout the
walls of the small intestine and large intestine, and occasionally extending into the lumen, were dozens of semi-firm, pale tan-yellow nodules which ranged from 0.3 to 2 cm in diameter. On cut section, these nodules were pale tan and bulged slightly. The mesentery was tan and diffusely moderately thickened.
Laboratory Results:
Qualitative fecal analysis: Few Capillaria sp. eggs, few Heterakis sp. eggs, few coccidial oocysts
Microscopic Description:
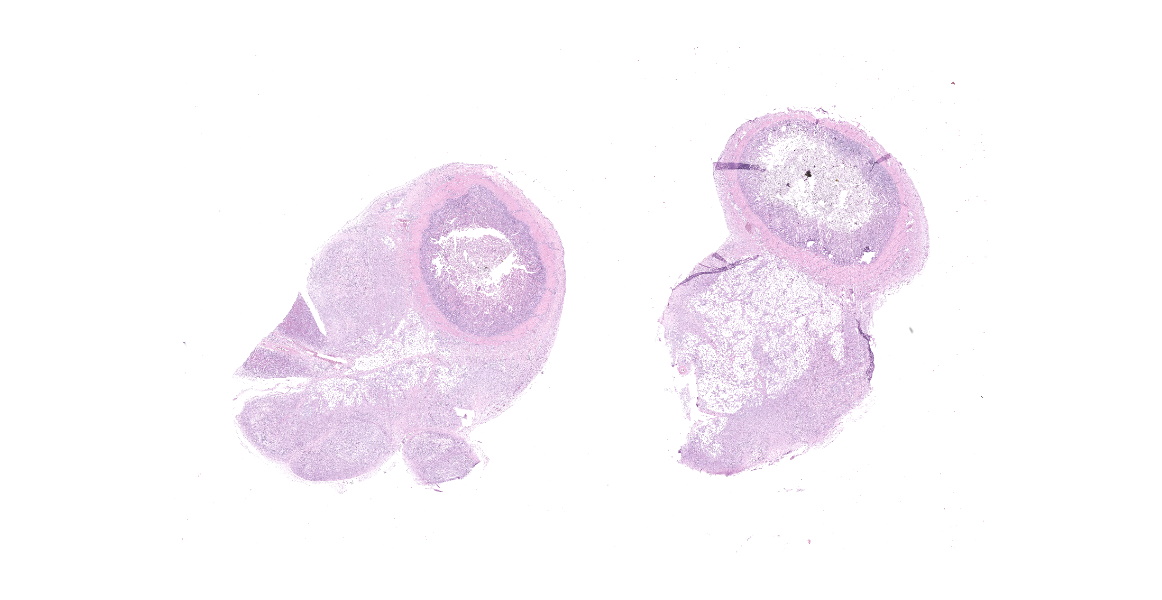
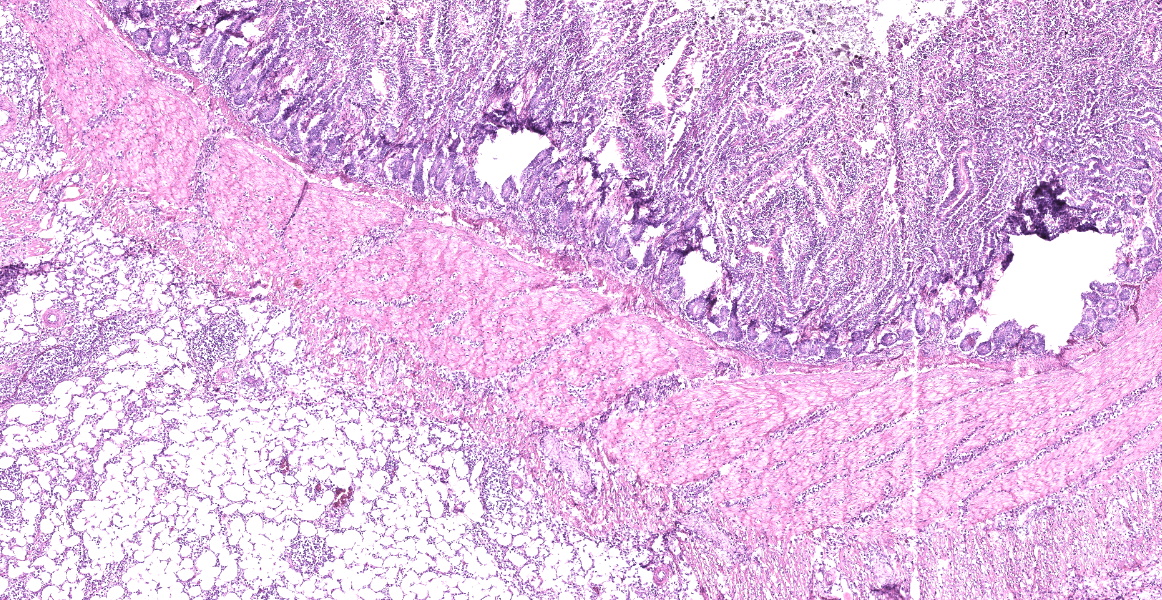
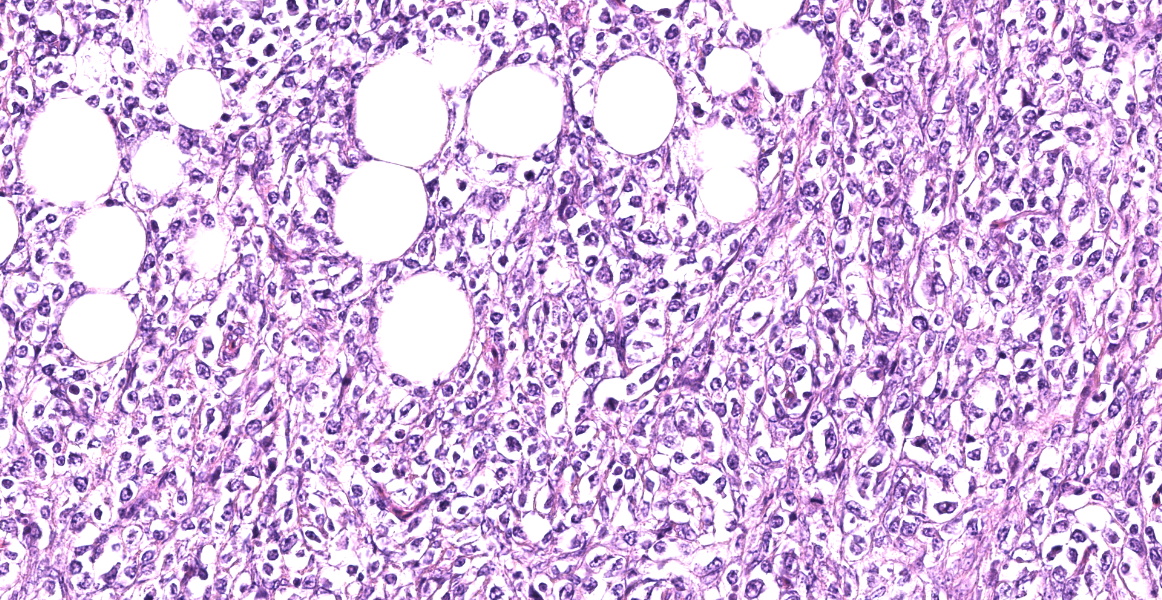
Small intestine: Diffusely expanding and effacing the serosa of the small intestine and the surrounding mesentery is a poorly demarcated, highly cellular proliferation of neoplastic round cells. Neoplastic cells are arranged in sheets and supported by collagenous stroma. Neoplastic cells are round with distinct cell borders and have small to moderate amounts of eosinophilic cytoplasm. Nuclei are round, finely stippled to vesiculate and have 1-3 nucleoli. There is moderate anisocytosis and anisokaryosis and 43 mitoses in 10 high-power fields (400x). Multifocally, neoplastic cells infiltrate the small intestinal muscularis and submucosa. The adjacent pancreas is extensively obscured to effaced by similar neoplastic cells.
Immunohistochemistry:
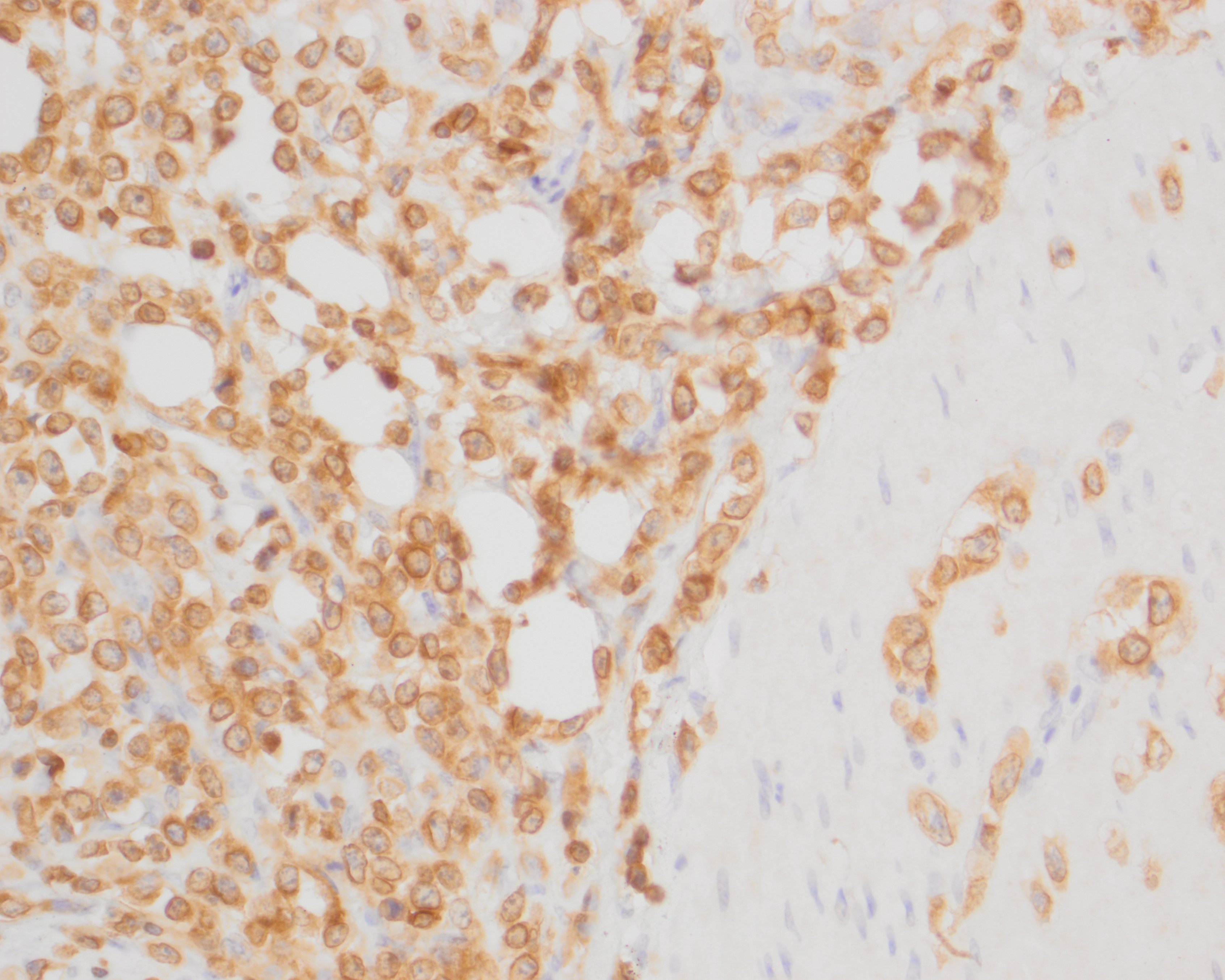
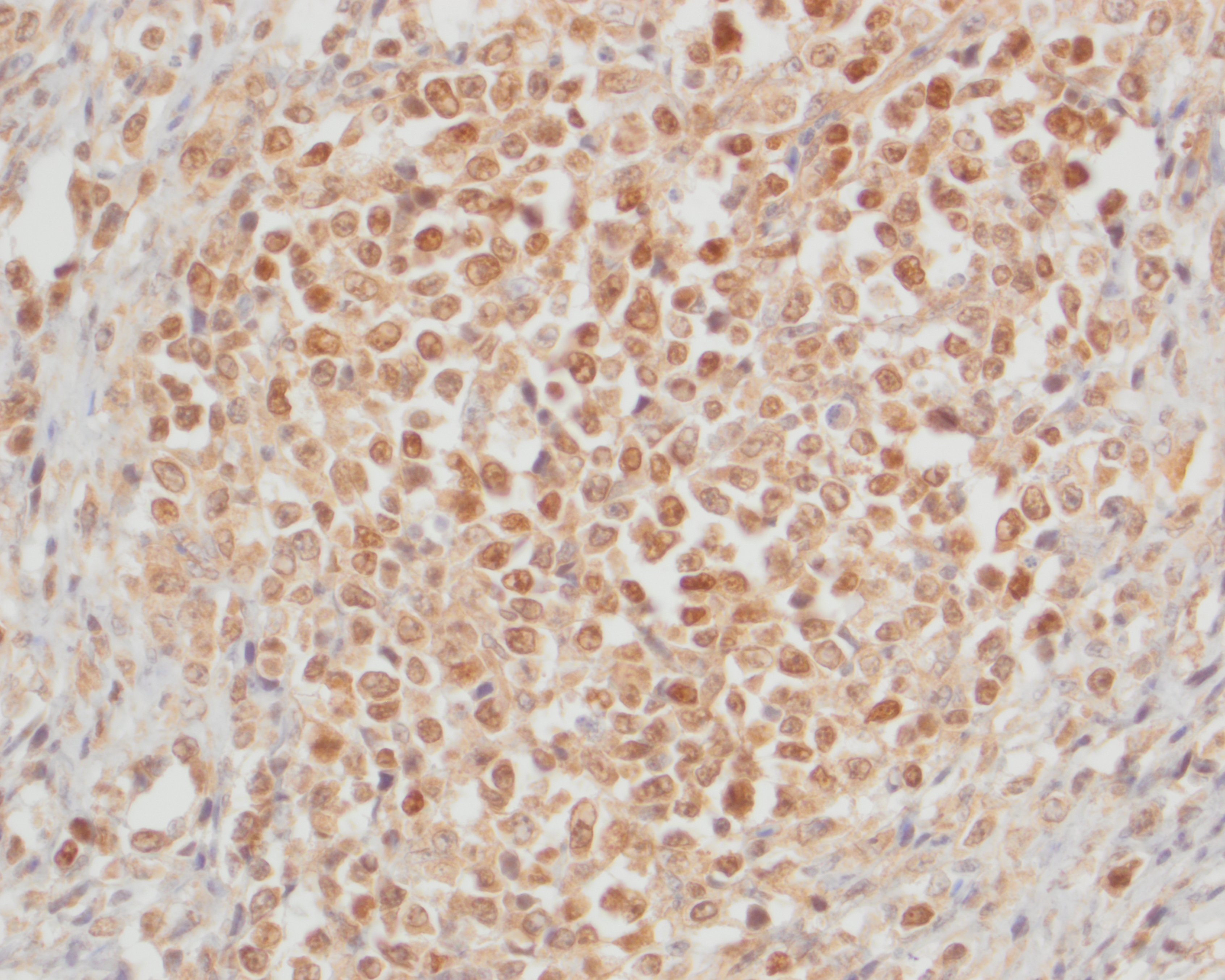
The neoplastic round cells within the small intestine, mesentery and pancreas exhibit strong perimembranous immunoreactivity for CD3, strong nuclear immunoreactivity for Meq and are not immunoreactive for BLA.36.
Contributor's Morphologic Diagnoses:
Small intestine: T-cell lymphoma
Pancreas: T-cell lymphoma
Contributor's Comment:
The histopathologic findings are consistent with visceral lymphoma, which in this case was immunohistochemically confirmed as Marek's disease using antibodies directed against the Marek's disease virus Meq protein.
Marek's disease (MD) in chickens occurs upon inhalation of gallid alphaherpesvirus-2, a cell-associated alphaherpesvirus with lymphotropic properties similar to gammaherpesviruses.13 Marek's disease virus (MDV) is a member of the genus Mardivirus and are divided into three serotypes: Gallid herpesvirus 2 (MDV-1), Gallid herpesvirus 3 (MDV-2), and Meleagrid herpesvirus 1 (MDV-3). Of these three serotypes, only MDV-1 causes disease in chickens.13,15 Strains of MDV are classified into four pathotypes based on their virulence, referred to as mild (m), virulent (v), very virulent (vv) and very virulent plus (vv+) strains.1,13 The m strains can cause mild neurological disease, the v and vv strains can cause lymphomas, and the vv+ strains can cause fatality with severe brain edema and tumors in unvaccinated and vaccinated chickens.1,15
Previously, MDV posed a serious economic threat causing up to 60% mortality in layer flocks, and currently exists in all poultry-producing countries.2,13,14 Despite the more recent advent and widespread use of a live-attenuated vaccine, sporadic losses still occur on individual farms, and the disease is still a concern in poultry flocks.13 Concerns of the vaccine selecting for MDV strains of higher virulence also exist, as MDV vaccines prevent clinical signs but do not prevent virus replication and shedding in vaccinated chickens.1,14 Nearly all chickens can be infected by MDV and develop tumors, and turkeys, quail, and pheasants are also susceptible to infection and disease.13 Marek's disease outbreaks may occur in unvaccinated chickens as young as 3-4 weeks, although most serious cases begin after 8-9 weeks of age.13
The pathogenesis of MDV infection in vivo is divided into four stages.15 In the early cytolytic phase, B cells undergo cytolytic infection.15 In the latency phase, MDV infects CD4+ and CD8+ T-cells.15 Following MDV reactivation in CD4+ T-cells, a late cytolytic and immunosuppressive phase is initiated.15 Finally, the transformation phase occurs and is characterized by the development of CD4+ T-cell lymphomas.15
Marek's disease can manifest as distinct lymphoproliferative syndromes, such as lymphomas, cutaneous leukosis, atherosclerosis, early mortality, cytolytic syndromes, immunosuppression, and lymphoproliferation in the eyes and CNS as well as peripheral nerves resulting in 'fowl paralysis'.13 Gross lesions associated with MD may include unilaterally gray or yellow, edematous, and enlarged peripheral nerves, such as the sciatic and brachial nerves, which may be 2 to 3 times normal size.13 Lymphomas may also occur in virtually all visceral organs, including the mesentery, intestine, pancreas, kidney, liver, spleen, bursa of Fabricius, thymus, iris ("gray eye"), feather follicles, heart, muscle and gonads, particularly the ovary.13 Visceral tumors may appear as gray or yellow firm nodules or diffuse organ enlargements.13 In this case, differential diagnoses for the gross lesions would include avian tuberculosis or Hjarre's disease (coligranuloma), which were later ruled-out with microscopic examination.
Microscopic lesions within visceral organs correspond to proliferations of monomorphic neoplastic small to large T-cells, lymphoblasts and mononuclear inflammatory infiltrates.13 Within peripheral nerves, lymphoproliferative lesions are separated into types A and B.3,13 Type A lesions are neoplastic and consist of T-cells and fewer B-cells.3,13 Type B lesions are inflammatory and are characterized by mononuclear cell infiltrates composed of small lymphocytes and plasma cells and fewer macrophages.3,13
In the current case, only lymphoma affecting visceral organs was appreciated. In addition to infiltrating and obliterating the intestinal walls, mesentery, and pancreas, similar neoplastic lymphocytes extensively effaced the ovary and infiltrated the splenic capsule.
The primary differential diagnoses for lymphoma in chickens include Marek's disease, and the retroviruses avian leukosis virus (ALV) and reticuloendothelial virus (REV).7,13 Immunophenotyping with T- and B-cell markers can help to distinguish between MDV and ALV, as MDV commonly induces T-cell lymphomas, while ALV induces B-cell lymphomas.7 In the current case, neoplastic cells exhibited strong perimembranous labeling for the T-cell marker CD3 and were negative for the B-cell marker BLA.36. PCR may also be used to identify the presence of MDV.6,7,11
In addition, immunohistochemistry using antibodies directed against the MDV-specific viral antigens Meq and pp38 can be used to help confirm a diagnosis of MD. Meq (Marek's EcoQ) is a leucine zipper regulatory protein similar to the Fos and Jun oncoproteins, and has been demonstrated to be critical to MD oncogenesis.10,14 The functions of Meq include DNA binding, chromatin remodeling and regulation of transcription.14 Meq can form homodimers or heterodimers with proto-oncoproteins, such as c-Myc, c-Fos, ATF and c-Jun. Meq can also bind to and sequester RB, p53 and cyclin-dependent kinase 2 (CDK2), leading to dysregulated cell-cycle control and the oncogenic transformation of T-cells.14 The exact biological function of pp38 is currently unknown, but has been associated with lymphoid tropism, oncogenicity, reactivation from latency, viral replication in the early lytic phase and maintenance of transformation in MDV-infected tumor cells.8 Meq has been shown to be the only viral antigen consistently expressed, but pp38 positive cells may also be observed.7 In our case, the neoplastic T-cells exhibited strong nuclear immunoreactivity for Meq but were not immunoreactive for pp38.
Contributing Institution:
NIH Comparative Biomedical Scientist Training Program, National Cancer Institute
https://nih-cbstp.nci.nih.gov/
JPC Diagnosis:
- 1. Small intestine, mesentery, pancreas: Lymphoma.
- 1. Small intestine, lumen: Cestode adult.
JPC Comment:
In 1907, Hungarian veterinarian Jozsef Marek described a syndrome of polyneuritis in chickens termed fowl paralysis and now known as Marek's disease.1 In 1967, the herpesviral etiology was discovered. Observations that the virus spread via indirect contact and remained infectious in the environment for months led to further research into the transmission of the virus, and in 1970, Calnek et al. described enveloped herpesvirus particles in the feather follicular epithelium that were infectious to and produced Marek's disease in other chickens.4 Since then, cell-free virions produced in the feather follicle epithelium and shed in feathers and dander of infected birds have been recognized as the source of horizontal transmission.
The economic impact of Marek's disease is estimated to be up to $2B USD annually, with losses stemming from decreased productivity, immunosuppression leading to comorbidities, and aesthetic condemnation at slaughter.1,5,12 As such, tremendous research has gone into developing prevention and control methods, and there are currently three main lines of effort: selecting for genetically resistant chickens, improving biosecurity to prevent spread between flocks, and vaccination. Several Marek's disease virus (MDV) vaccinations have been developed, including a vaccine using herpesvirus of turkeys and attenuated MDV strains SB1 and CVI988.5 While these vaccines are generally effective in preventing clinical disease in vaccinated birds, they do not produce sterile immunity, and vaccinated birds can still be infected and shed the virus. This has selected for and supported the spread of more virulent strains of MDV that would, in unvaccinated populations, be self-limiting in scope due to their high mortality rates. 1,5,12
Recent research has uncovered one key component of MDV transmission: conserved herpesvirus protein kinase (CHPK).9 CHPKs are encoded by all members of the Herpesviridae family and support a variety of functions throughout viral infection stages (such as nuclear egress and viral DNA replication).9 In MDV, CHPK is not required for cell-to-cell transmission or progression of clinical disease, but it is specifically required for horizontal transmission.9 Recent studies indicate that CPHK is most likely integral for production of cell-free virions, as mutated CPHK results in defects in the viral replication pathway in feather follicular epithelium, but CPHK's role in establishing infections in new individuals has not been ruled out.9 In either case, this research provides insight into the horizontal transmission of MDV which has yet to be overcome by vaccination strategies.
References:
- Bertzbach LD, Conradie AM, You Y, Kaufer BB. Latest insights into Marek's disease virus pathogenesis and tumorigenesis. Cancers. 2020;12(3):647.
- Boodhoo N, Gurung A, Sharif S, Behboudi S. Marek's disease in chickens: a review with focus on immunology. Veterinary research. 2016;47(1):1-9.
- Burgess SC, Basaran BH, Davison TF. Resistance to Marek's disease herpesvirus-induced lymphoma is multiphasic and dependent on host genotype. Veterinary Pathology. 2001;38(2):129-42.
- Calnek BW, Adldinger HK, Kahn DE. Feather follicle epithelium: A source of enveloped and infectious cell-free herpesvirus from Marek's disease. Avian Dis. 1970; 14:219-233.
- Davidson I. Out of Sight, but Not Out of Mind: Aspects of the Avian Oncogenic Herpesvirus, Marek's Disease Virus. Animals. 2020; 10: 1319-1332.
- Gimeno IM, Dunn JR, Cortes AL, El-Gohary AE, Silva RF. Detection and differentiation of CVI988 (Rispens vaccine) from other serotype 1 Marek's disease viruses. Avian diseases. 2014;58(2):232-43.
- Gimeno IM, Witter RL, Fadly AM, Silva RF. Novel criteria for the diagnosis of Marek's disease virus-induced lymphomas. Avian Pathology. 2005;34(4):332-40.
- Gimeno IM, Witter RL, Hunt HD, Reddy SM, Lee LF, Silva RF: The pp38 Gene of Marek's Disease Virus (MDV) Is Necessary for Cytolytic Infection of B Cells and Maintenance of the Transformed State but Not for Cytolytic Infection of the Feather Follicle Epithelium and Horizontal Spread of MDV. Journal of Virology2005;79(7):4545-4549.
- Krieter A, Ponnuraj N, Jarosinski KW. Expression of the Conserved Herpesvirus Protein Kinase (CHPK) of Marek's Disease Alphaherpesvirus in the Skin Reveals a Mechanistic Improtance for CHPK during Interindividual Spread in Chickens. J Virol. 2020; 95(5):1-12.
- Lupiani B, Lee LF, Cui X, Gimeno I, Anderson A, Morgan RW, Silva RF, Witter RL, Kung HJ, Reddy SM. Marek's disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proceedings of the National Academy of Sciences. 2004;101(32):11815-20.
- Mete A, Gharpure R, Pitesky ME, Famini D, Sverlow K, Dunn J. Marek's disease in backyard chickens, a study of pathologic findings and viral loads in tumorous and nontumorous birds. Avian diseases. 2016;60(4):826-36.
- Nair V. Spotlight on avian pathology: Marek's Disease. Avian Path. 2018; 47(5):440-442.
- Nair V, Gimeno I, Dunn J. Neoplastic diseases: Marek's disease. In: Swayne DE, ed. Diseases of Poultry. 14th Wyley Blackwell. 2020.
- Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek's disease virus: from miasma to model. Nature Reviews Microbiology. 2006;4(4):283-94.
- Shi MY, Li M, Wang WW, Deng QM, Li QH, Gao YL, Wang PK, Huang T, Wei P. The emergence of a vv+ MDV can break through the protections provided by the current vaccines. Viruses. 2020;12(9):1048.