Signalment:
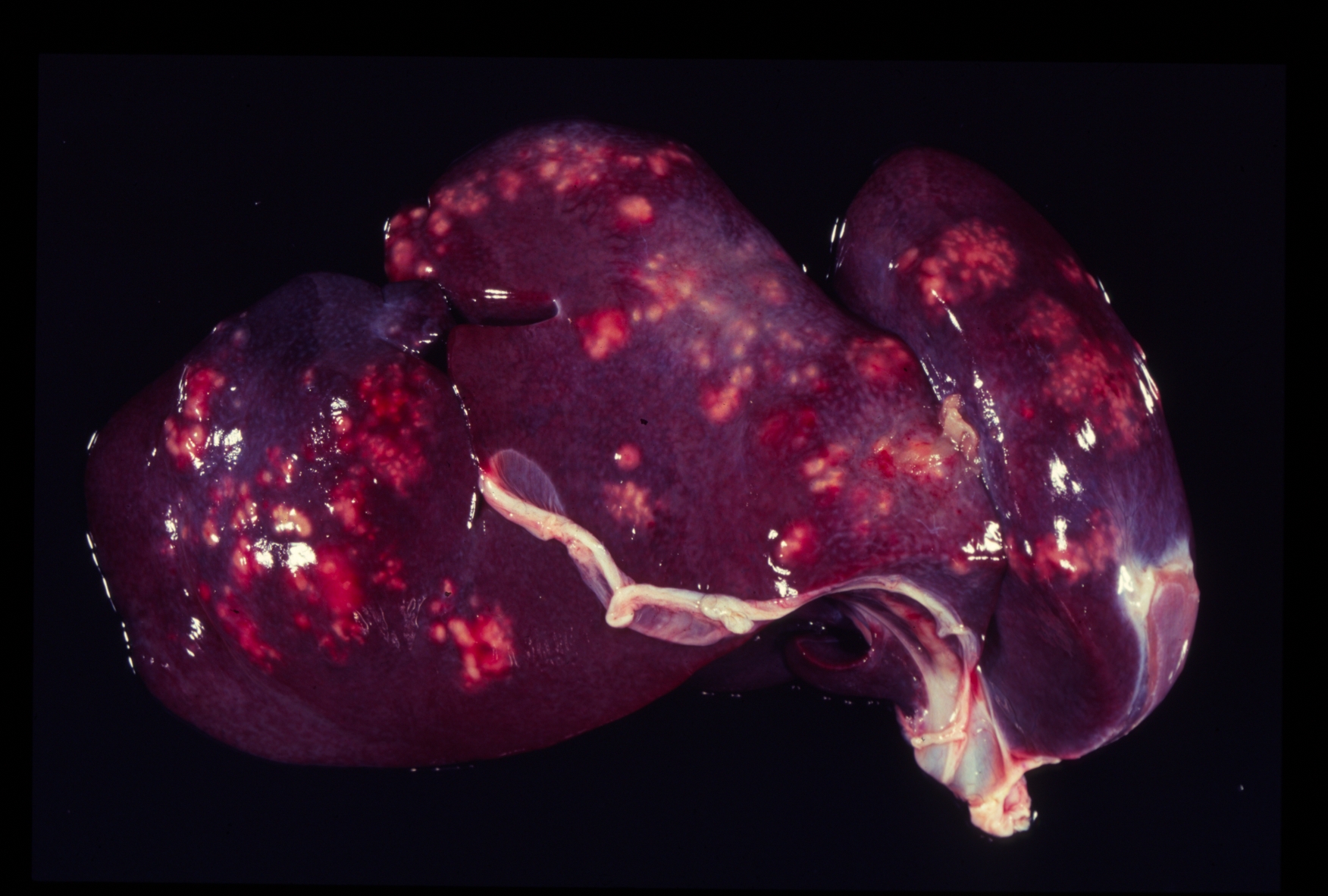
Gross Description:
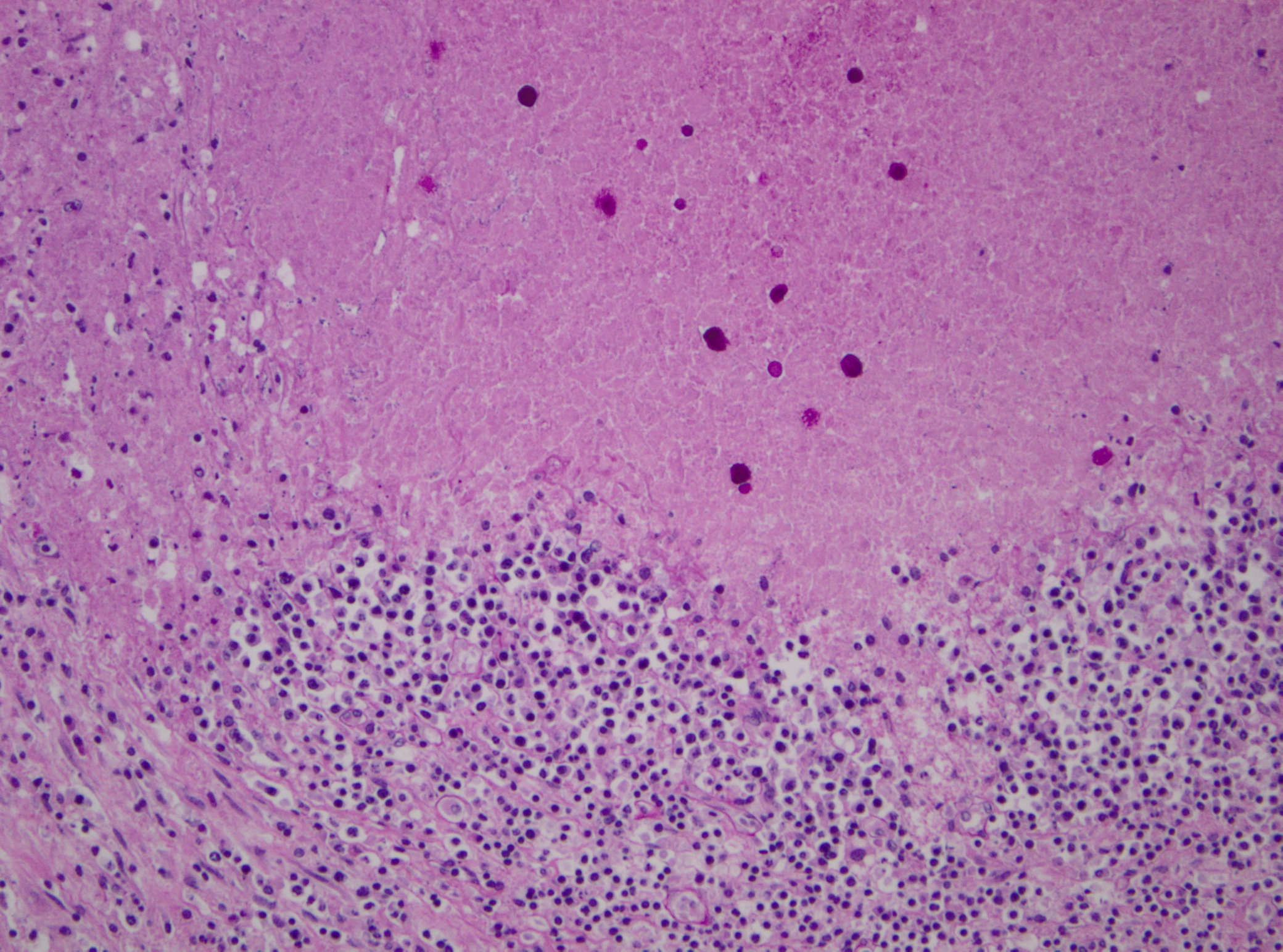
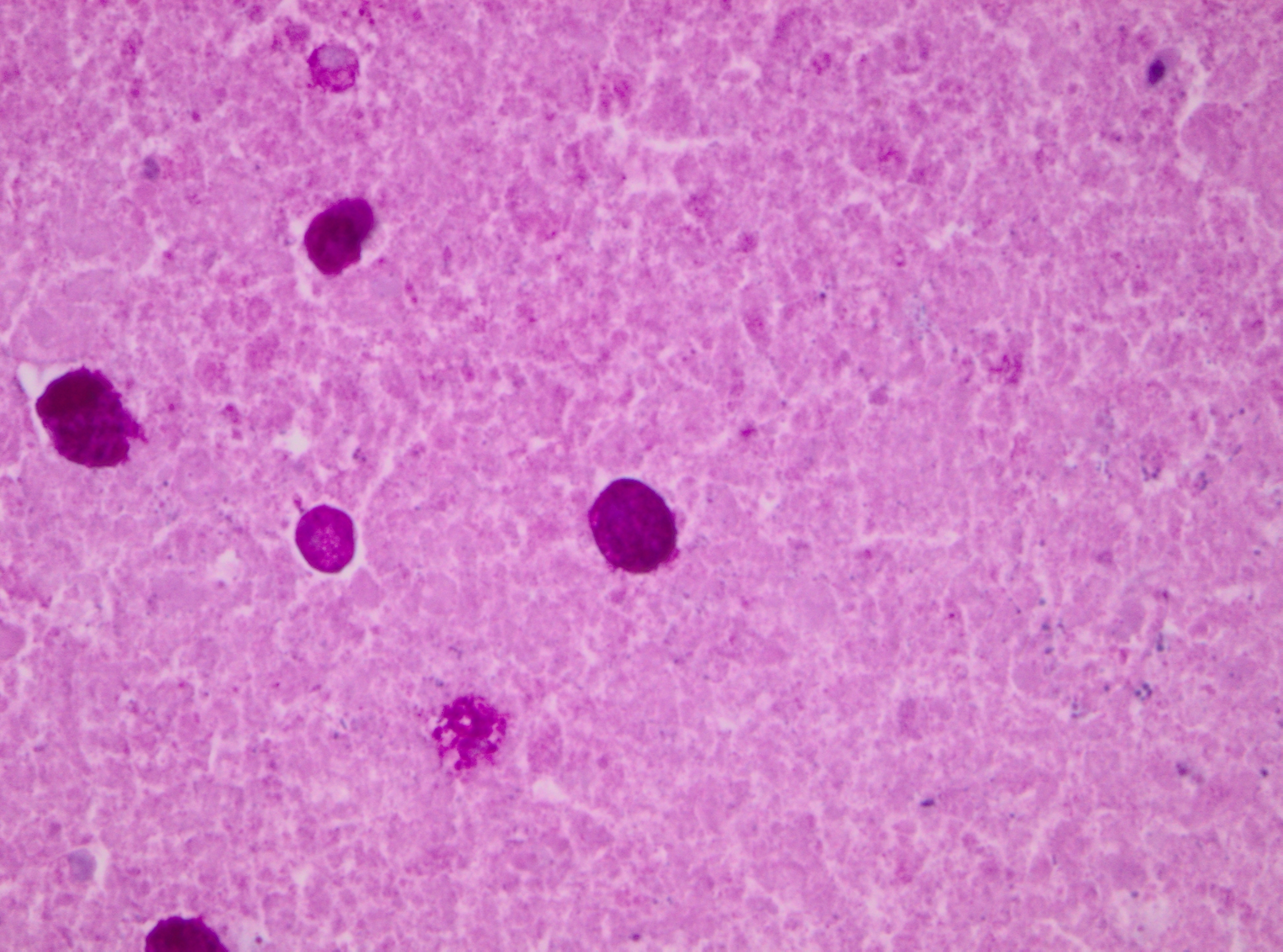
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
The liquid of the anterior eye chamber contained a urea concentration of 19.98 mmol/L (120 mg/dL). Aerobic and anaerobic microbiological cultures of the liver resulted in a mild amount of α-hemolytic Streptococcus spp. and coagulase-negative Staphylococcus spp. Aerobic and anaerobic microbiological cultures of the stomach revealed a mild amount of Enterococcus species, coagulase-negative Staphylococcus spp., coryneform bacteria, α- hemolytic Streptococcus spp., Geotrichium species, Prevotella bivia and Prevotella intermedia. Both organs were culturally negative for Listeria monocytogenes under cold enrichment.
PCR of the liver was negative for human hepatitis virus A, B, C, and Francisella tularensis.
Immunohistology of the liver was positive for Entamoeba histolytica and negative for Toxoplasma gondii, Coxiella burnetti and Listeria monocytogenes.
Condition:
Contributor Comment:
Infection with E. histolytica occurs via the fecaloral route. In human patients, excystation takes place in the large intestine. The trophozoites are usually commensals within the intestinal lumen, reproducing by binary fission and encysting as they move further down the digestive tract. Cysts are shed for many months to years in untreated humans. The cysts remain infective for weeks to months in a moist environment.(13,19) A complete intra-intestinal life cycle, including cyst formation, is only reported to occur in humans and non-human primates; therefore, the zoonotic potential of infected non-primate mammals is thought to be limited.(18)
Pathogenicity of E. histolytica is affected by the strain of organism, host species infected, nutritional status, environmental factors and bacterial flora of the gastrointestinal tract.(20) Only in cases of mucosal invasion, which happens in less than 10% of human patients, does E. histolytica become pathogenic and lead to amoebic dysentery. (13,19) Clinical signs in affected monkeys include apathy, lethargy, weakness, dehydration, gradual weight loss, anorexia, vomiting, and severe diarrhea, which may be catarrhalic or hemorrhagic.20 Amoebiasis commonly presents as necrotizing colitis in humans and many non-human primates.(5,13,19,20) However, fibrino-necrotizing gastritis seems to be the principal lesion in certain species of leaf-eating monkeys of the subfamily colobinae [old world monkeys (Cercopithecoidea), family cercopithecidae], including colobus monkeys (Colobus guereza), silvered leaf monkeys (Presbytis cristatus), dusky leaf monkeys (Presbytis obscurus), and proboscic monkeys (Nasalis larvatus).(4,8-11) The stomach of the colobinae is divided into four parts: presaccular, saccular, tubular, and pyloric portion, with the first two portions serving as enlarged fermentation chambers as a special adaption to the leaf-eating lifestyle. It is suggested that the normal neutral pH within these gastric compartments provides a favourable environment for excystation of ingested E. histolytica cysts, followed by tissue invasion.(4,8) Invasive trophozoites are regularly detected within the ulcerative gastric lesions.(4,8-11) In rare cases, and independently of whether the primary lesion is in the stomach or in the colon, some trophozoites are thought to enter the vessels of the mesenteric vasculature, thereby leading to metastatic foci of amoebic infection in distant parts of the body.(20) Fatal amoebiasis with abscess formation, particularly in the liver and more infrequently the lung and the central nervous system, is reported in man, baboons, chimpanzees, orang-utans, spider monkeys, douc langurs, and several colobus monkeys.(4,5,8,11,13,18-20) Amoebic hepatic lesions may become clinically obvious many years after the initial exposure and without concurrent intestinal lesions and fecal cyst shedding.(8,13,18) Similar to the presented case, most reported cases of amoebic hepatic lesions in non-human primates were characterized by a multifocal distribution and granulomatous and necrotizing lesions.(4,8,11,18) The central necrotic areas of the lesions are thought to represent foci of caseous necrosis due to the action of lytic substances produced by the trophozoites.(8) In human patients, however, the liver lesions are mainly situated in the right liver lobe and consist of a single abscess in most patients (65-75%). (13)
The differential diagnosis of granulomatous and necrotizing hepatic lesions in primates includes yersiniosis, salmonellosis, listeriosis, tularemia, tuberculosis, necrobacillosis, Q-fever, histoplasmosis, yellow fever, infections with lymphocytic choriomeningitis virus (callitrichid hepatitis), human hepatitis B virus, herpesvirus simiae (herpesvirus B), herpesvirus saimiri, simian varicella virus, herpesvirus tamarinus, herpes simplex virus, cytomegalovirus, ebola virus, toxoplasmosis, Capillaria hepatica, and schistosomiasis. Non-human primates are possible reservoir hosts of human hepatitis A virus; however, they display only slight hepatocellular degeneration, necrosis and inflammation and are most likely clinically asymptomatic. Furthermore, chimpanzees have been experimentally infected with the human hepatitis C virus; however, spontaneous infections of non-human primates have not been reported.(1) Due to the zoonotic potential of most of these diseases, increased personal protection and a thorough etiologic work-up is suggested in cases of hepatitis in non-human primates.
JPC Diagnosis:
Conference Comment:
These virulence factors must enable E. histolytica to overcome a variety of innate host defenses. The first and most extensive host barrier is the layer of mucin extending from the oral cavity to the rectum. The glycoproteins found in host mucin competitively bind to residues used in amoebic attachment; resident intestinal bacteria also bind to mucin, further reducing available binding sites to the protozoan. The complement system plays a role in the innate immune response to E. histolytica. The neutral cysteine proteinase elaborated by the parasite has been shown to activate complement via the classical and alternative pathways, thus activating the immune response. However, the Gal/GalNAc lectin possessed by the organism binds C8 and C9, thus preventing formation of the membrane attack complex.(12)
In addition to lectin-mediated evasion of the complement system, E. histolytica has several methods by which it evades the host immune system. The cysteine proteinases and hydrolytic enzymes not only degrade extracellular matrix proteins, they also degrade IgA and IgG antibodies, thereby facilitating initial invasion of the intestinal epithelium as well as aiding in systemic dissemination.(12) When immune complexes bind to the amoeba, a unique process of capping takes place whereby the bound antibodies are quickly moved to the posterior pole of the organism, the cap is released and the plasma membrane is regenerated;(7) thus, immune complexes are essentially shed from the protozoal cell surface. E. histolytica is also able to alter the host acute-phase immune response, primarily via an unknown mechanism of T-cell- modulated macrophage function, especially Th1.(12) The amoeba also produce monocyte locomotion-inhibitory factor which inhibits the respiratory burst in macrophages. Research also has demonstrated the presence of surface perioxiredoxin which neutralizes host-generated reactive oxygen species and nitric oxide.(7) Serine proteases secreted by E. histolytica bind to cathepsin G secreted by neutrophils, and protozoal arginase consumes host L-arginine, a precursor for the nitric oxide production by host macrophages.(7)
References:
2. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed., vol. 2. Philadelphia, PA: Elsevier Ltd; 2007:3-296.
3. Crisostomo-Vazquez M del P, Jimenez-Cardoso E, Arroyave-Hernandez C. Entamoeba histolytica sequences and their relationship with experimental liver abscesses in hamsters. Parasitol Res. 2006;98:94-98.
4. Frank H. Pathology of amoebiasis in leaf monkeys (colobidae). Proceedings 24th Int Symp Dis Zoo Anim 1982:321-326.
5. Fremming BD, Vogel FS, Benson RE, Young RJ. A fatal case of amebiasis with liver abscesses and ulcerative colitis in a chimpanzee. J Am Vet Med Assoc. 1955;126:406-407.
6. Gardiner CH, Fayer R, Dubey JP. Amoebae. In: Gardiner C, Dubey JP, eds. An Atlas of Protozoan Parasites in Animal Tissues. 2nd ed. Washington, DC: Armed Forces Institute of Pathology; 1998.
7. Lejune M, Rybicka J, Chadee K. Recent discoveries in the pathogenesis and immune response toward Entamoeba histolytica. Future Microbiol. 2009;4:105-118.
8. Loomis MR, Britt JO, Gendron AP, Holshuh HJ. Hepatic and gastric amebiasis in black and white colobus monkeys. J Am Vet Med Assoc. 1983;183:1188-1191.
9. Muller R, Ruedi D. Gastric amebiasis in a proboscic monkey (Nasalis larvatus). Acta Zoolog Pathol Antverpiensia. 1981;76:9-16.
10.Palmieri PR, Dalgard DW, Connor DH. Gastric amebiasis in a silvered leaf monkey. J Am Vet Med Assoc. 1984;185:1374-1375.
11.Pang VF, Chang CC, Chang WF. Concurrent gastric and hepatic amebiasis in a dusky leaf monkey (Presbytis obscurus). J Zoo Wildl Med. 1993;24:204-207.
12.Petri, WA Jr. Intestinal invasion by Entamoeba histolytica. Subcell Biochem. 2008;47:221-232.
13.Pritt BS, Clark CG. Amebiasis. Mayo Clin Proc. 2008;83:1154-1160.
14.Shimada A, Muraki Y, Awakura T, et al. Necrotic colitis associated with Entamoeba histolytica infection in a cat. J Comp Pathol. 1992;106:195-199.
15.Stedman NL, Munday JS, Esbeck R, Visvesvara GS. Gastric amebiasis due to Entamoeba histolytica in a Dama wallaby (Macropus eugenii). Vet Pathol. 2003;40:340-342.
16.Takano J, Narita T, Tachibana H, et al. Entamoeba histolytica and Entamoeba dispar infections in cynomolgus monkeys imported into Japan for research. Parasitol Res. 2005;97:255-257.
17.Takeuchi A, Jervis HR, Phillips BP. Electron-microscope studies of experimental Entamoeba histolytica infection in guinea pig .3. Histolysis of Cecum. Virchow's Archiv B-Cell Pathology Including Molecular Pathology. 1977;24:263-277.
18.Tammer R, M+�-�tz-Rensing K, Rolle S, Kaup FJ. A case of hepatic amebiasis in a colobus guereza monkey. Primate Rep. 2002;63:21-26.
19.Tannich E. The laboratory diagnosis of Entamoeba histolytica infections. J Lab Med. 2004;28:491-497.
20.Toft JD, Eberhard ML. Protozoan parasites, B. Sarcodines: Amoeba. In: Bennett BT, Henrickson R, eds. Nonhuman Primates in Biomedical Research. San Diego, CA: Academic Press; 1998:116-119.
21.Walsh JA. Problems in recognition and diagnosis of amebiasis - estimation of the global magnitude of morbidity and mortality. Rev Inf Dis. 1986;8:228-238.
22.Wittnich C. Entamoeba histolytica infection in a German shepherd dog. Can Vet J. 1976;17:259-263.
23.World Health Organization: Amoebiasis. Weekly Epidemiological Record 1997;72:97-99.