Signalment:
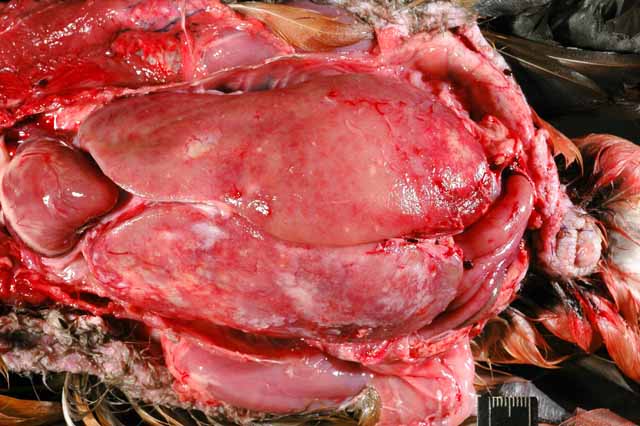
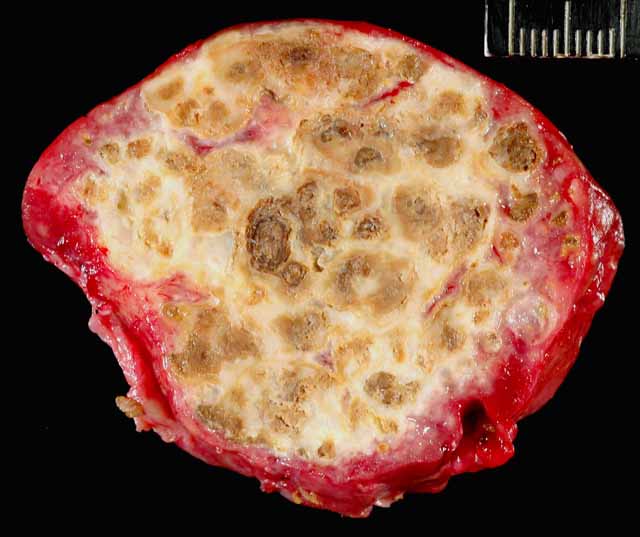
Gross Description:
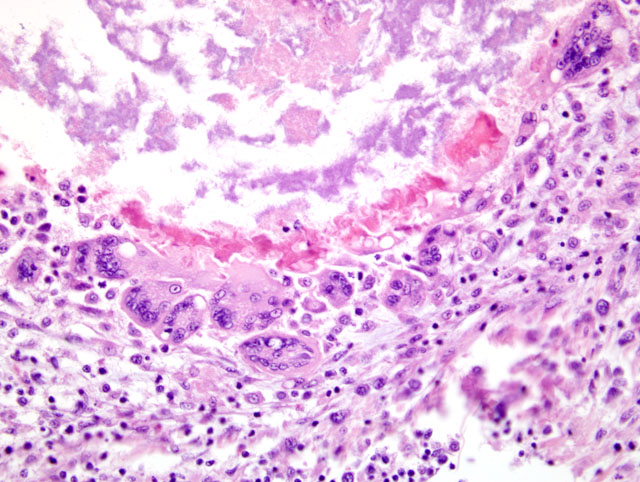
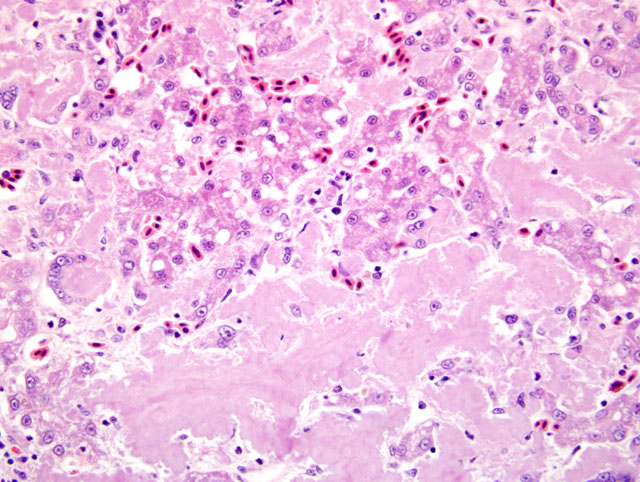
Histopathologic Description:
Morphologic Diagnosis:
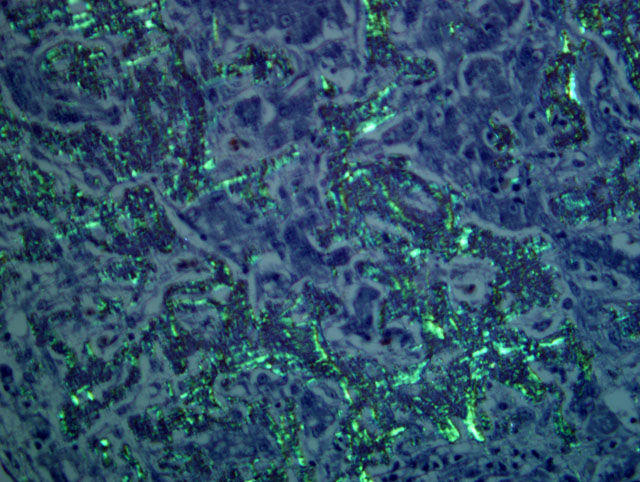
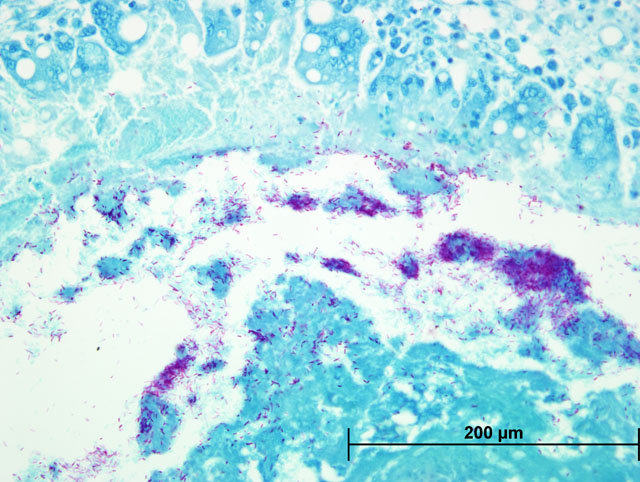
Liver: Severe multifocal to coalescing granulomatous hepatitis with intralesional acid-fast rod bacteria (Fig. 3-6) (Mycobacterium avium complex)
Liver: Severe amyloidosis
Lab Results:
Both Ziehl-Neelsen and Fites stains showed very large numbers of thin, variable length, acid-fast rods admixed with the debris at the center of the granulomas and occasionally within the surrounding multinucleated giant cells. These bacteria were gram positive with a Brown & Brenn stain. Immunohistochemical staining for Bacillus Calmette-Guerin (BCG) showed abundant amorphous immunoreactive material within the granulomas. The organism was identified as Mycobacterium avium complex by HPLC at the National Jewish Medical Center.
Condition:
Contributor Comment:
The term amyloid encompasses a group of biochemically distinct proteins with a similar beta-pleated sheet conformation arranged in variable length, 7.5-10 nm wide, nonbranching filaments (visible by electron microscopy) which give amyloid its characteristic Congo red staining and green birefringence, as well as its fluorescence with thioflavin-T or S.(1,4,5) At least 17 amyloid proteins have been characterized in humans and animals, with AA (derived from serum amyloid A), AL (derived from immunoglobulin light chains), and Aβ (β-amyloid protein found in cerebral Alzheimer disease lesions) being the most common in humans.(1) Of these, except for a single report of β-amyloid in cerebral vessels of an aged woodpecker, (7) only AA-amyloidosis has been reported in birds.(5) The precursor protein for AA, serum amyloid A (SAA), is a soluble acute phase response protein synthesized in the liver in response to inflammation via cytokines Il-1, Il-6, and TNF.(1,4,5) The mechanisms by which insoluble derivatives of SAA are deposited and accumulate are poorly understood, but because of its association with persistently elevated SAA concentrations, as occurs in chronic inflammatory conditions, this type of amyloidosis is known as secondary, or reactive, amyloidosis.
Among birds, amyloidosis has been reported in most orders, but is particularly common in captive Anseriformes where incidences may be almost 80% in ducks and 50% in geese and swans examined at necropsy.(5) In approximately 60-70% of amyloidosis cases in Anseriformes, an associated chronic inflammatory or infectious disease, such as mycobacteriosis, fungal disease, or enteric parasites, can be identified.(2,5,10) It has also been associated with bumblefoot in Pekin ducks and can be induced experimentally in ducks and chickens with injections of a variety of bacterial and adjuvant components.(5) The cases in which systemic amyloidosis is present without inflammation may be considered idiopathic or a result of nonspecific stresses associated with environmental conditions. For example, a study in white Pekin ducks free of chronic disease and parasites showed that increased crowding corresponded to increased rate and incidence of development of amyloidosis.(3) Amyloidosis has been seen in approximately 20% of avian mycobacteriosis cases overall, (6) and in up to 50-60% of the cases in Anseriformes.(2,8)
Mycobacteriosis in birds is predominantly caused by Mycobacterium avium-intracellulare (MAI) complex organisms or Mycobacterium genavense and is spread by fecal-oral transmission (rarely aerogenous) from environmental contamination.(9) They have low zoonotic potential, except to immunocompromised individuals, who are nevertheless more likely to acquire the infection from a common environmental source.(9) Some studies have found disproportionate susceptibility of waterfowl, especially perching ducks, to Mycobacterium sp. in zoologic collections.(9) The infection in this duck was systemic with severe involvement of the liver and scattered granulomas present in the spleen, on the serosal surface of the intestinal tract, and in the fascia of the cervical and syringeal regions. It was identified as a Mycobacterium avium complex species.Â
JPC Diagnosis:
1. Liver: Granulomas, multiple, with acid fast bacilli, etiology consistent with Mycobacterium sp.
2. Liver: Amyloidosis, diffuse, severe, with moderate hepatocellular atrophy, loss, degeneration, and necrosis and multifocal, moderate granulomatous hepatitis
Conference Comment:
References:
2. Brassard A: Amyloidosis in captive Anseriformes. Can J Comp Med Vet Sci 29:253-258, 1965
3. Cowan DF, Johnson WC: Amyloidosis in the white Pekin duck. Lab Invest 23:551-555, 1970
4. King NW, Alroy J: Intarcellular and extracellular depositions, degenerations: Amyloidosis. In: Veterinary Pathology eds., Jones TC, Hunt RD, King NW, 6th ed., pp. 50-54. Lippincott Williams & Wilkins, Baltimore, 1997
5. Landman WJM, Gruys E, Gielkens ALJ: Avian amyloidosis. Avian Pathol 27:437-449, 1998
6. Montali RJ, Bush M, Thoen CO, Smith E: Tuberculosis in captive exotic birds. JAVMA 169:920-927, 1976
7. Nakayama H, Katayama KI, Ikawa A, Miyawaki K, Shinozuka J, Uetsuka K, Nakamura SI, Kimura N, Yoshikawa Y, Doi K: Cerebral amyloid angiopathy in an aged great spotted woodpecker (Picoides major). Neurobiol Aging 20:53-56, 1999
8. Saggese MD, Riggs G, Tizard I, Bratton G, Taylor R, Phalen DN: Gross and microscopic findings and investigation of the aetiopathogenesis of mycobacteriosis in a captive population of white-winged ducks (Cairina scutulata). Avian Pathol 36:415-422, 2007
9. Tell LA, Woods L, Cromie RL: Mycobacteriosis in birds. Rev Sci Tech Off Int Epiz 20:180-203, 2001
10. Zschiesche W, Linke RP: Immunohistochemical characterization of spontaneous amyloidosis in captive birds as AA-type, using monoclonal and polyclonal anti-AA antibodies against mammalian amyloid. Acta Histochem 86:45-50, 1989