CASE 1: M19-03419 (4141690-00)
Signalment: Adult, gender unspecified, Red Angus (Bos taurus), bovine.
History: Feedlot cattle with acute onset respiratory signs with bilateral hemorrhagic nasal discharge. Two dead and five showing clinical signs out of a herd of 200. A few others had also died with similar signs in the past few months. Has been vaccinated against Mannheimia haemolytica.
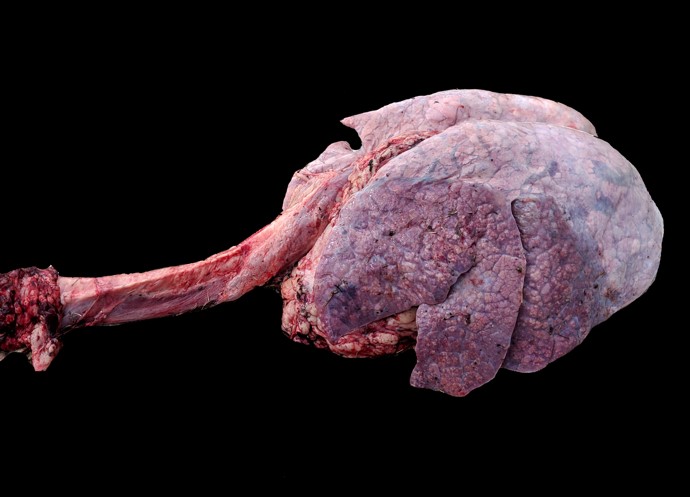
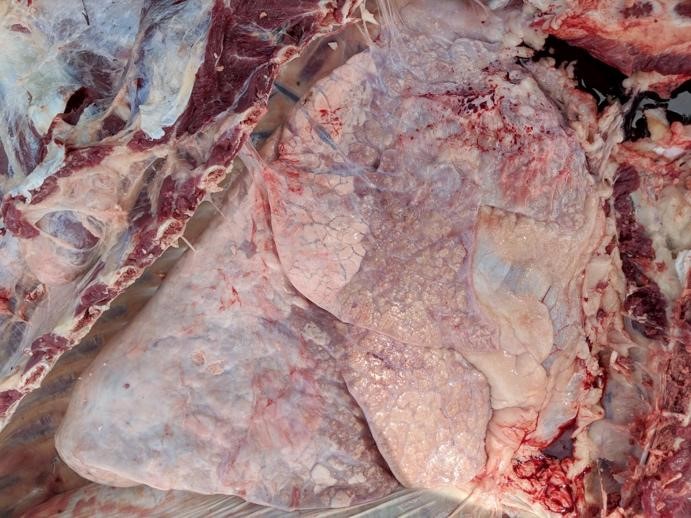
Gross Pathology: Cranioventral lung lobe consolidation with multifocal to coalescing, well-demarcated, pale yellow, slightly raised, variably sized nodules over the pleural surface. On cut surface, the lesions extend into the parenchyma. There are multifocal emphysema and multiple fibrous adhesions to thoracic cavity wall.
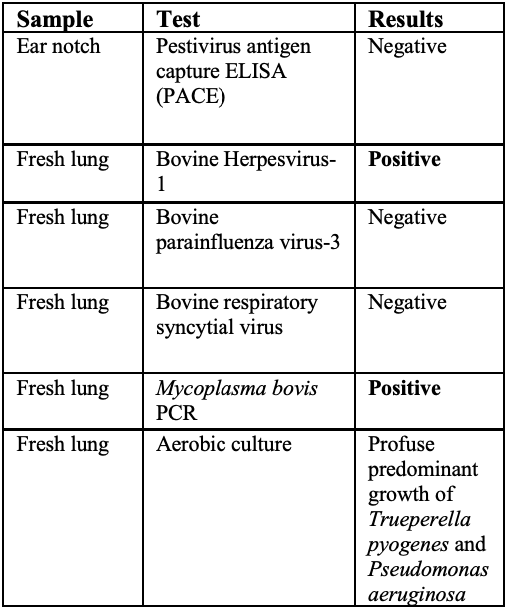
Laboratory results:
See table.
Microscopic description:
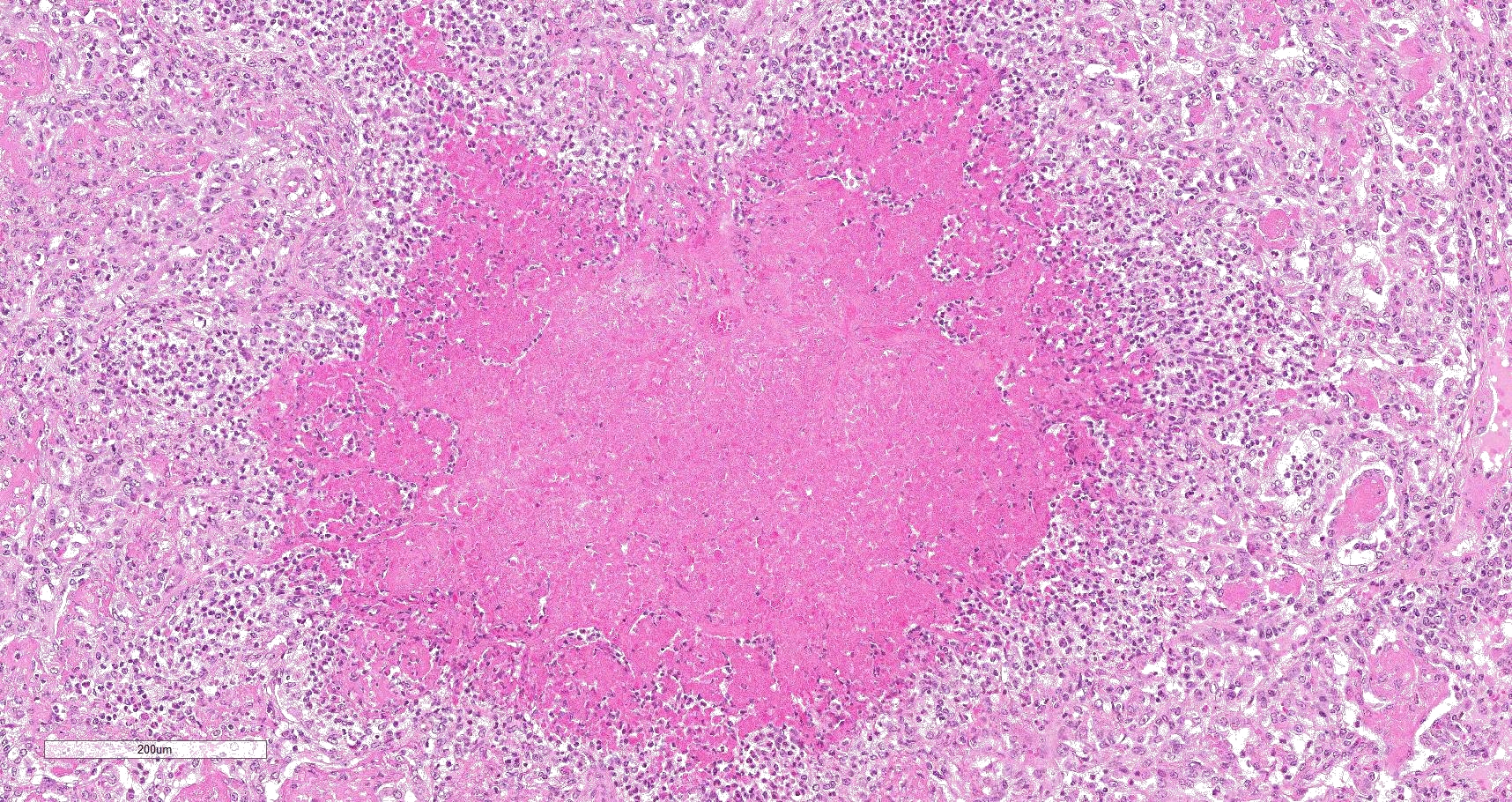
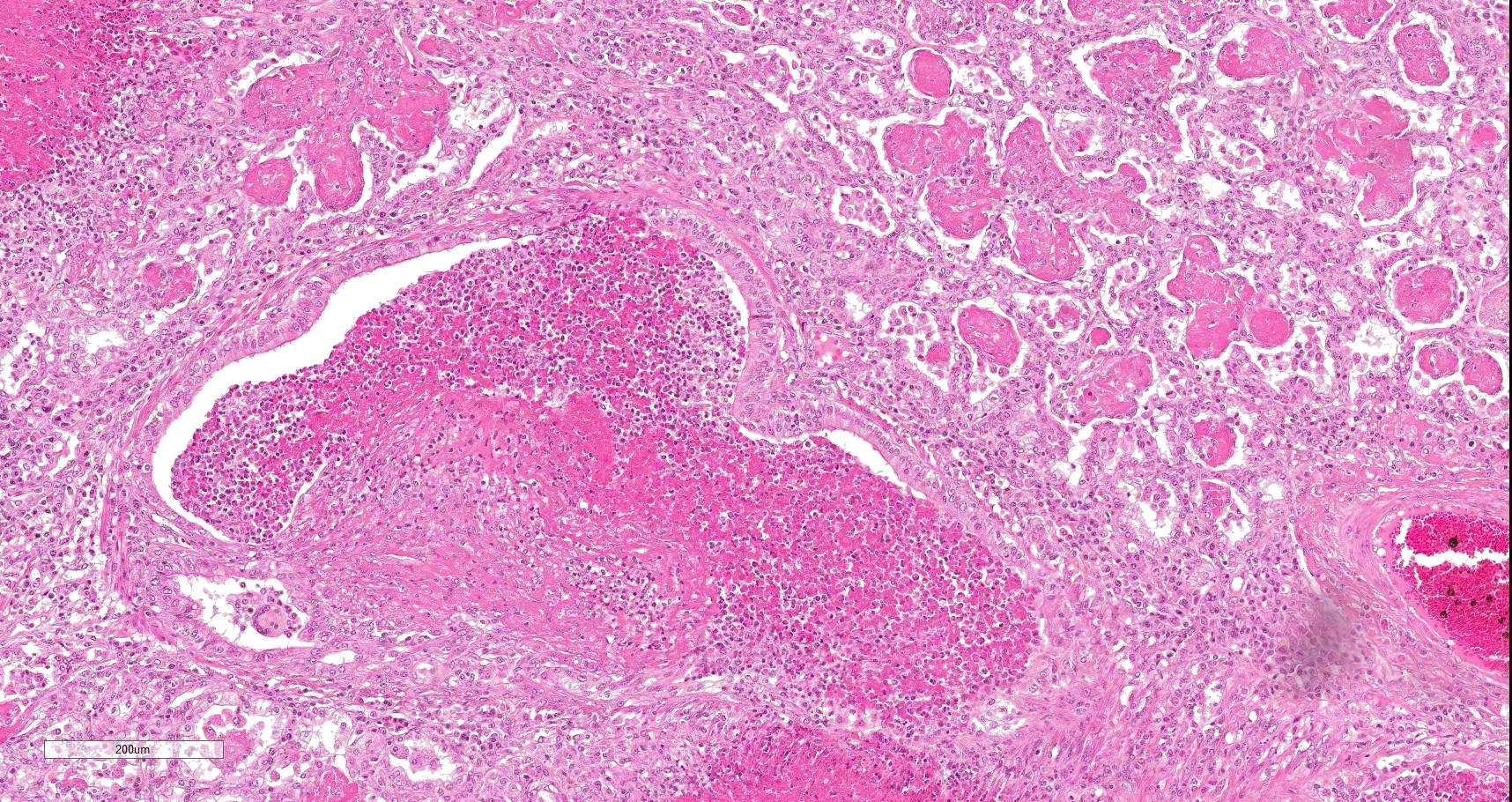
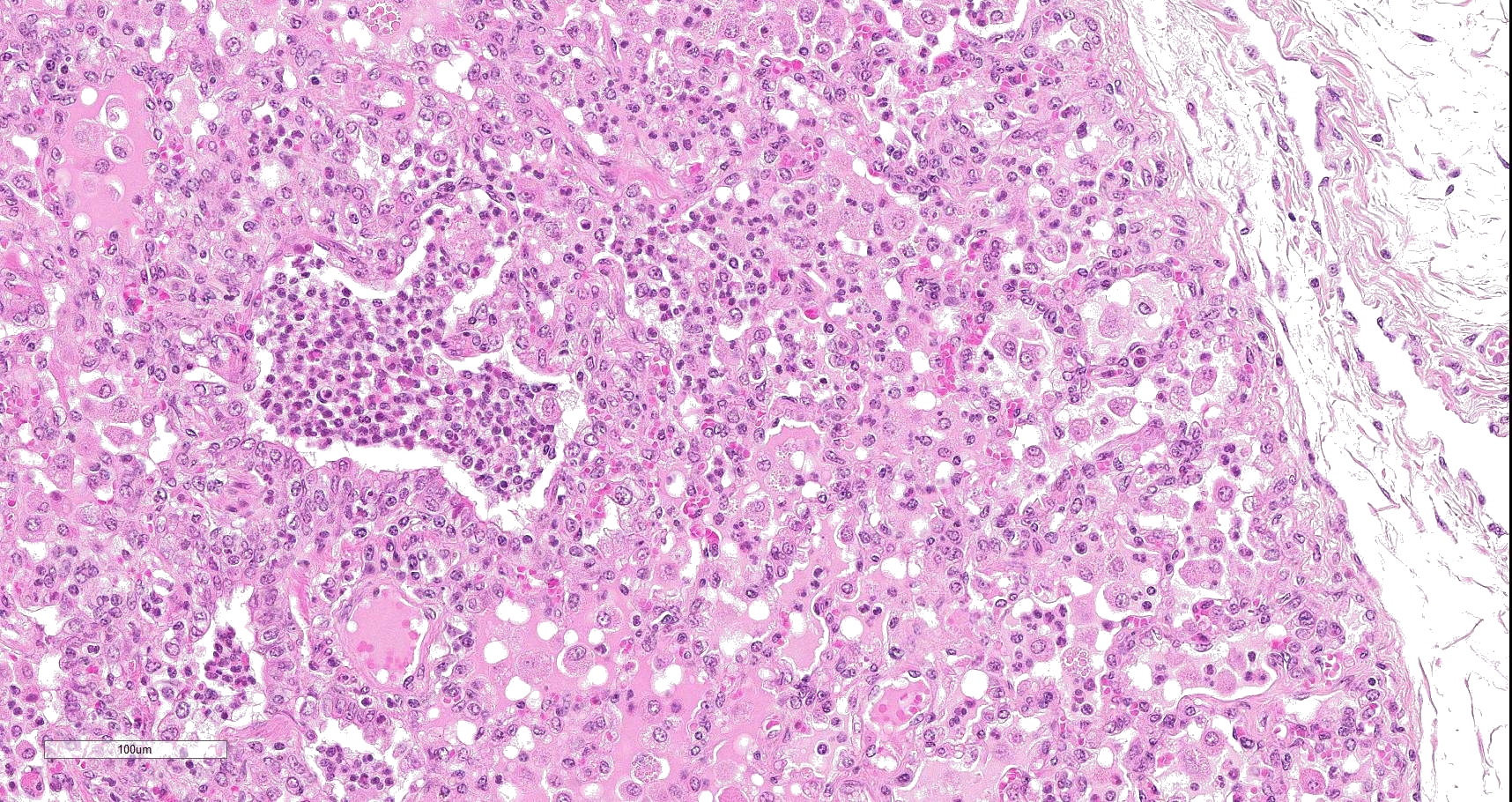
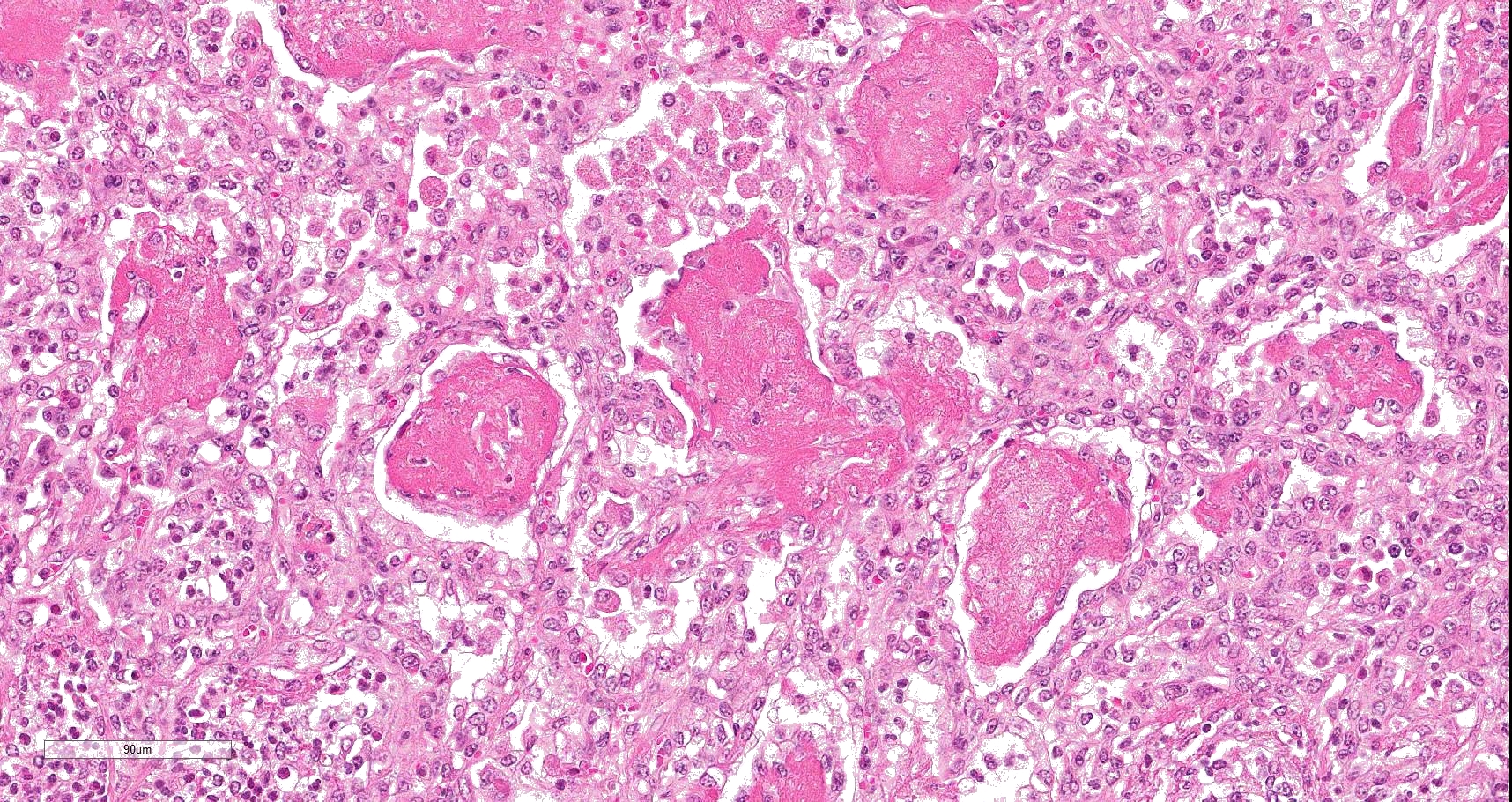
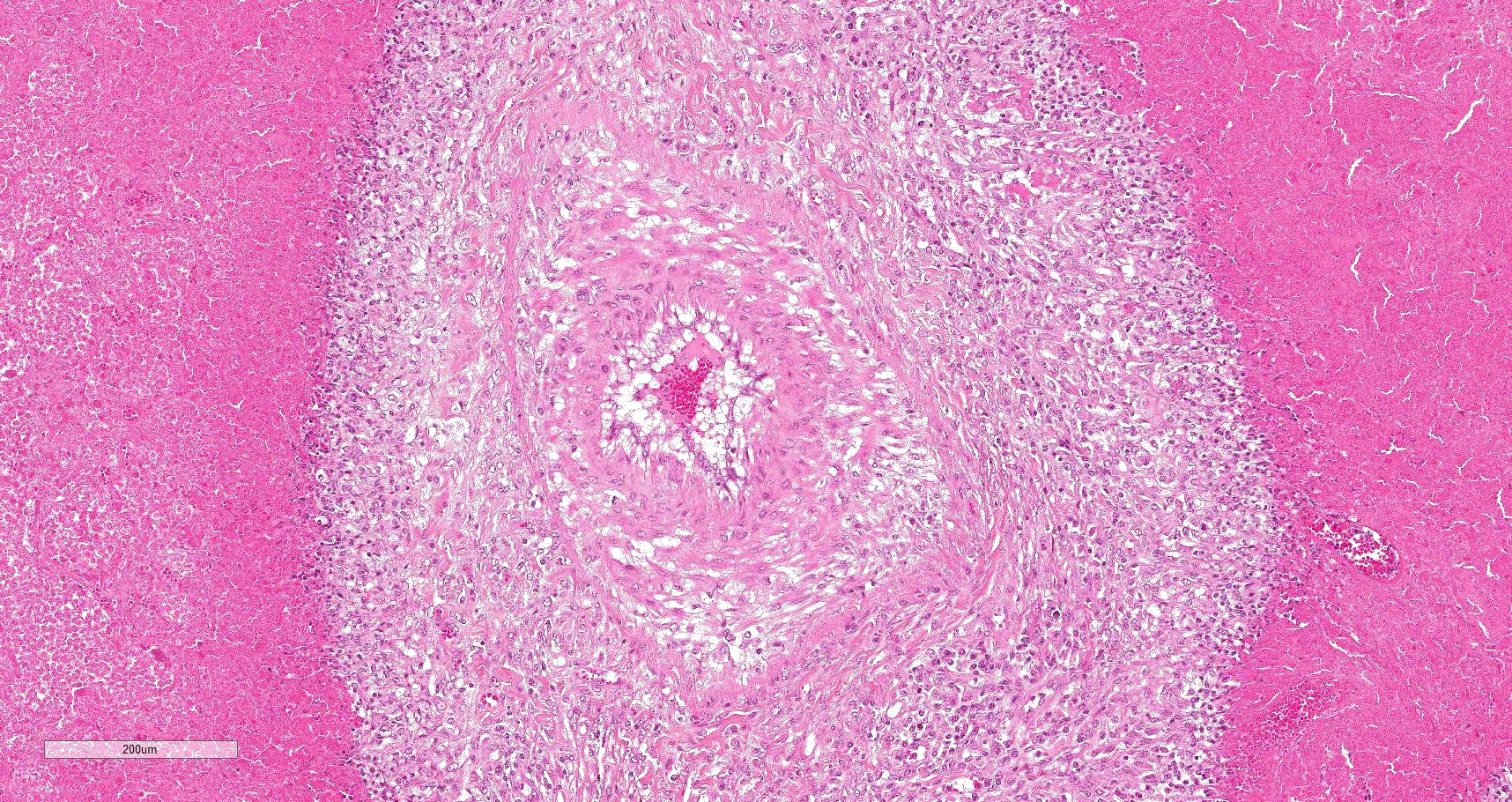
Multifocally to coalescing, mainly centered on bronchi and bronchioles are large areas of accumulation of eosinophilic debris, along with necrotic leukocytes that have lost their nuclei but retained their cellular outline (ghostlike remnants of leukocytes) (caseous necrosis) with occasional mineralization that is surrounded by variable amounts of neutrophils, a rim of macrophages with scattered lymphocytes, and in some areas, mild fibroblast proliferation. The remaining bronchi and bronchioles epithelium are attenuated, hyperplastic and lumina filled with eosinophilic and inflammatory debris, predominantly neutrophils. There are many areas with type II pneumocyte hyperplasia, and multifocally, the alveolar lumina are filled with homogenous to eosinophilic strands (fibrin) or paler eosinophilic fluid (edema), along with increased alveolar macrophages and neutrophils. The interlobular septa are moderately expanded by edema and have multiple lymphatic vessels and veins with large thrombi, some of which are attached to the vessel wall and have undergone reorganization. The pleura is mildly thickened with edema.
Lesions are similar in submitted slides, although with some variation among the sections.
Contributor's morphologic diagnosis:
Lungs: Bronchopneumonia, caseonecrotizing, subacute, multifocal to coalescing, marked; with type II pneumocyte hyperplasia, and pleural and interlobular edema and thrombosis.
Contributor's comment:
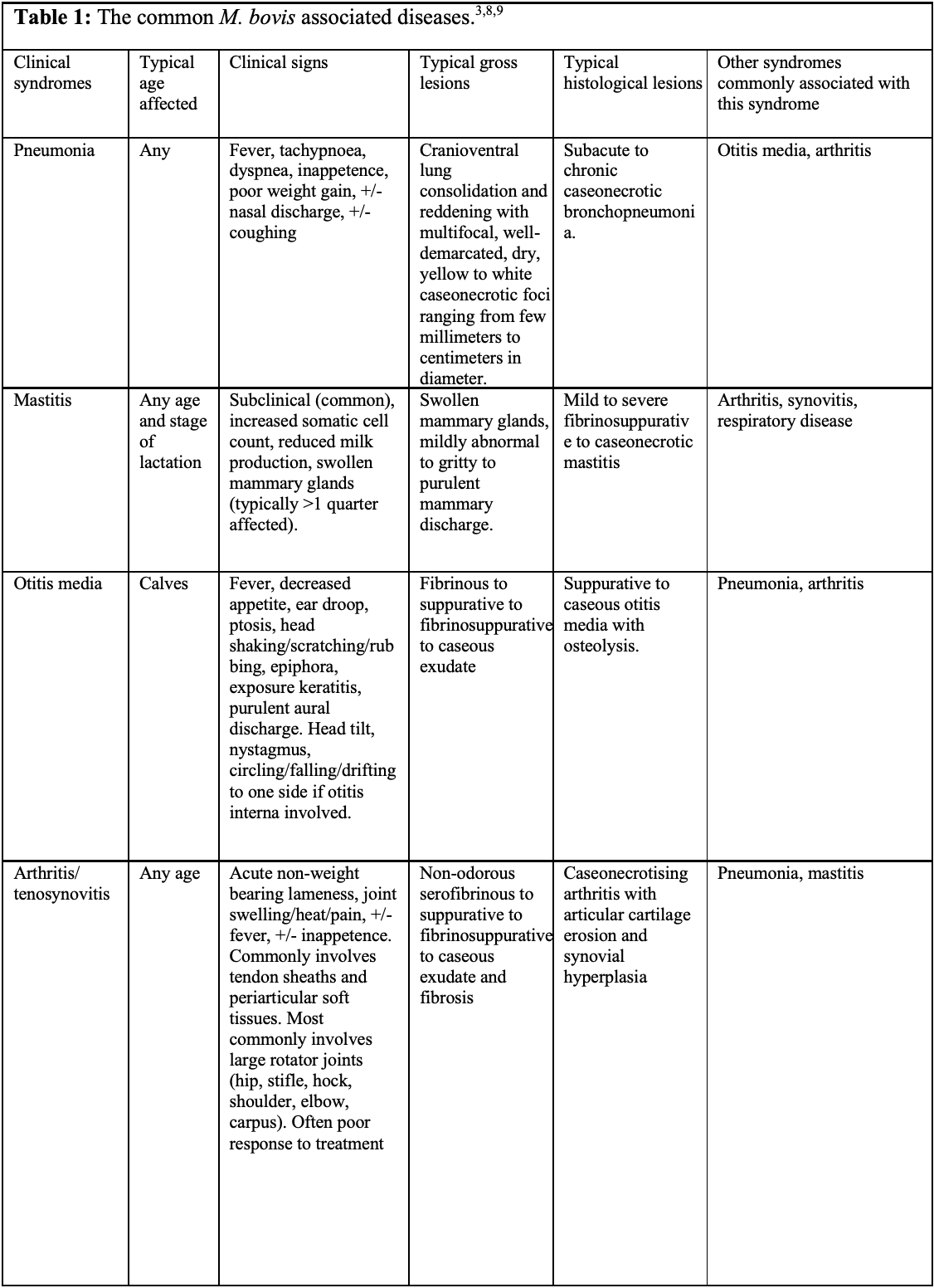
Mycoplasma bovis has significant economic and welfare consequences as it frequently results in chronic disease that fails to respond to antibiotics.10 Some animals can be asymptomatic and shed for months to years, sometimes intermittently, with increased shedding associated with stressful events such as comingling, transportation and entry into feedlot.1,10 Asymptomatic shedders are likely the major source of infection and transmission is via nasal secretions, aerosols, ingestion of contaminated milk, and less importantly via fomites (e.g. contaminated water, feed, housing, so forth).3,9,10 The pathogen is capable of causing various diseases, which have been collectively termed Mycoplasma bovis-associated diseases.9 These include pneumonia, mastitis, otitis media and arthritis/tenosynovitis (see table 1).1,3,9,10 Although M. bovis is known to cause chronic caseonecrotic bronchopneumonia, its role in other forms of acute and chronic pneumonia remains incompletely understood.1,2,10 Other less common or less established clinical syndromes include decubital abscesses, keratoconjunctivitis sicca, meningitis, myocarditis/endocarditis, genital infections and abortions.1,3,9,10 M. bovis infection can concurrently result in one or more of these syndromes.3,9,10
In this case, a diagnosis of M. bovis bronchopneumonia was made based on characteristic histological changes and a positive PCR. Diagnosis of M. bovis cannot be made by isolation or detection of the agent alone, as clinically healthy animals can shed the pathogen.1,2,9,10 Typical gross findings include cranioventral bronchopneumonia with red discoloration and consolidation and multiple well-demarcated, white to yellow caseonecrotic foci that ranges from several millimeters to centimeters in diameter.2,3,9 Classical histological lesions include suppurative bronchopneumonia with characteristic well-demarcated foci of caseous necrosis centered around bronchi and bronchioles, with amorphous eosinophilic material, along with ghostlike remnants of leukocytes, that are surrounded by neutrophils, followed by a rim of lymphocytes, plasma cells, macrophages and fibroblasts, which were features seen in this case.2,3,8,9 In this case Trueperella pyogenes and Pseudomonas aeruginosa were also cultured from the lung. These two agents were considered secondary opportunistic agents and may have resulted in the histological changes that are not normally associated with M. bovis pneumonia, such as the large thrombus within the interlobular septa.
M. bovis is able to colonize mucosal surfaces, persist at sites of disease and evade host clearance despite eliciting strong immune responses, resulting in chronic disease.2,10 The exact pathogenesis is not well understood, but the direct and indirect immunomodulatory effects on inflammatory cells, as well as the large family of variable surface lipoproteins (Vsps) are thought to be the major contributors.2,9,10 Vsps undergo high frequency size and phase variation, allowing for strain variation and large antigenic variation, which have been suggested to assist in humoral immune response evasion.1,2,3 Like other Mycoplasma species, M. bovis is able to adhere to mucosal surfaces, however unlike other Mycoplasma species, colonization does not result in ciliostasis.2 Despite extensive experimental research, there is still incomplete understanding behind the exact pathogenesis of M. bovis bronchopneumonia, including how they elicit lung damage, and the type of immune response that contributes to disease, as opposed to protection and clearance of M. bovis.2,10
M. bovis is thought to form synergism with other respiratory pathogens, including Mannheimia haemolytica, Pasteurella multocida and BoHV-1.1,9 Synergism with BVDV has also been suggested, but remains controversial with inconsistent results across different studies.3,12 It is also frequently associated with other infectious agents, including viral agents such as Bovine Respiratory Syncytial Virus, Bovine Parainfluenza Virus-3, Bovine Viral Diarrhea Virus (BVDV) and bacterial agents, most commonly Mycoplasma arginini and Trueperella pyogenes.1,2
In this case, BoHV-1 was also detected from the lung tissue by PCR. BoHV-1 can result in bronchointerstitial pneumonia with bronchiolar erosion, type II pneumocyte proliferation, and occasionally epithelial syncytia in alveoli, as well as eosinophilic intranuclear inclusion bodies.3 Apart from type II pneumocyte proliferation, these were not a feature in our case. The significance and role played by BoHV-1 in this case is unclear. BoHV-1 is considered to infect animals for life, and stress may cause reactivation of latent infections (e.g. from the M. bovis infection in this case).3 However, an alternative theory is BoHV-1 was a predisposing factor to the development of M. bovis bronchopneumonia. Respiratory viral agents such as BoHV-1 can result in lysis of ciliated epithelium, impairing the mucociliary apparatus, as well as reducing the function of alveolar macrophages, and has immunosuppressive effects including downregulation of type 1 interferon, leukocyte apoptosis and decreases MHC 1 and MHCII expression.3,4 Furthermore, experimentally, co-infection with BoHV-1 followed by M. bovis resulted in more severe clinical signs and lung lesions compared to M. bovis alone.12
Contributing Institution:
EMAI, Woodbridge road
Menangle, NSW, 2568, Australia
https://www.dpi.nsw.gov.au/about-us/services/laboratory-services/veterinary
https://www.dpi.nsw.gov.au/about-us/science-and-research/centres/emai
JPC diagnosis:
Lung: Bronchopneumonia, caseonecrotic, multifocal, severe, with diffuse interstitial pneumonia, and type II pneumocyte hyperplasia.
JPC comment:
The contributor describes Mycoplasma bovis well and summarizes the forms of disease in cattle. Recent research has increased our understanding of this bacteria, though much remains unknown.
While this disease may not have economic importance on the scale of BSE or FMD, bovine respiratory disease in U.S. feedlot cattle is responsible for approximately $55 million annually. Despite many outbreaks being associated with new exposure to infected cattle, there are no travel restrictions on movement related to M. bovis.11
Recent research has elucidated several mechanisms and attributes of M. bovis that contributes to its pathogenicity, including four adhesins (a-enolase, VpmaX protein, NADH oxidase, TrmFO protein), two nucleases (MBOVPG45_0215, MnuA), and a secretory nuclease (MBOV_RS02825). In vitro studies have also identified fructose 1,6-biphosphate aldolase and methylenetetrahydrofolate-tRNA-(uracil-5-)-methyltransferase as molecules that augment adhesion by binding plasminogen and fibronectin, respectively.11
Neutrophils stimulated by M. bovis do not produce NETs, and this effect is due to a reduction in reactive oxygen species production in neutrophils.5 However, the nucleases previously mentioned are associated with cytotoxicity and the degradation of neutrophil extracellular traps (NETs), when present.6,7
Extracellular DNA (eDNA) has been shown (in vitro) to be a limiting nutrient for the growth of M. bovis. When exposed to sufficient amounts of eDNA, the bacterium produces H2O2, which appears to be cytopathic for actively dividing cells. eDNA, an important component of biofilms, is often found in abundance in tissue due to necrosis, apoptosis, autophagy, pyroptosis, and extracellular release in vesicles. Importantly, this research has been performed in vitro only, testing against embryonal bovine lung cells. In vivo results may not correlate exactly.13
North American bison (Bison bison) develop infections from M. bovis as a primary pathogen, causing polyarthritis and/or pneumonia. However, the isolate is genetically different from the M. bovis isolated from cattle, and to date, the bison strain has not caused natural or experimental disease in cattle. The bison variant of M. bovis may represent a new host-adapted variant.11,12
References:
- Caswell JL, Archambault
M. Mycoplasma bovis pneumonia in cattle. Anim Health Res Rev. 2007; 8; 161-186.
- Caswell JL, Bateman KG, Cai
HY, Castillo-Alcala F. Mycoplasma bovis
in respiratory disease of feedlot cattle. Vet Clin
Food Anim. 2010; 26; 365-379.
- Caswell JL, Williams KJ. Respiratory system. In
Maxie MG, ed. Jubb, Kennedy and
Palmer's Pathology of Domestic Animals. 6th ed., vol. 2.
Philadelphia, PA: Saunders Elsevier; 2016: 552-554, 537-538.
- Ellis JA. Update on viral pathogenesis in BRD. Anim Health Res Rev. 2009; 10; 149-153.
- Gondaira S,
Higuchi H, Nishi K, Iwano H, Nagahata
H. Mycoplasma bovis escapes bovine
neutrophil extracellular traps. Veterinary Microbiology. 2017;199:68-73.
- Huang J, Zhu H, Wang J, et al. Fructose-1,6-bisphosphate aldolase is involved in Mycoplasma bovis colonization as a fibronectin-binding adhesin. Research in Veterinary Science. 2019;124:70-78.
- Josi C, Burki S, Vidal S, et al. Large-scale analysis of the Mycoplasma
bovis genome identified non-essential,
adhesion- and virulent-related genes. Frontiers in Microbiology.
2019;10:2085.
- López A,
Martinson SA. Respiratory system, mediastinum, and pleurae. In Zachary JF,
ed. Pathologic Basis of Veterinary Disease. 6th ed., St.
Louis, MO: Elsevier Mosby; 2017: pp.531-532.
- Maunsell FP,
Donovan GA. Mycoplasma bovis infections
in young calves. Vet Clin Food Anim. 2009; 25;
139-177.
- Maunsell FP, Woolums AR, Francoz D, et
al. Mycoplasma bovis infections in cattle.
J Vet Intern Med. 2011; 25: 772-783.
- Perez-Casal J.
Pathogenesis and virulence of Mycoplasma bovis.
Vet Clin Food Anim. 2020.
- Prysliak T, van
der Merwe J, Lawman Z, et al. Respiratory disease caused by Mycoplasma bovis is enhanced by exposure to bovine herpes
virus 1 (BHV-1) but not to bovine viral diarrhea
virus (BVDV) type 2. Can Vet J. 2011; 52; 1195-1202.
- Register KB, Olsen SC, Sacco RE, et al. Relative
virulence in bison and cattle of bison-associated genotypes of Mycoplasma
bovis. Veterinary Microbiology. 2018;222:55-63.
- Zhu X, Dordet-Frisoni
E, Gillard L, et al. Extracellular DNA: A nutritional trigger of Mycoplasma
bovis cytotoxicity. Frontiers in
Microbiology. 2019;10:2753.