WSC 22-23
Conference 21
Case I:
Signalment:
12-year-old, male neutered, Havanese dog (Canis familiaris)
History:
A 12-year-old, male neutered Havanese dog has been managed through the Neurology Service over the course of 2 years for a right forebrain mass and cluster seizures. The dog was treated with radiation therapy 2 years ago and continued to have seizures. More recently, the dog started showing progressive neurologic signs consistent with regrowth of the mass (more frequent seizures, circling to the right, dull mentation, decreased menace OS). The dog presented to the Neurology service for cluster seizures and was hospitalized for seizure management. The seizures could only be controlled with a valium CRI. His owners elected euthanasia given the persistent breakthrough seizure activity and progressive neurologic dysfunction.
Gross Pathology:
The right frontal lobe has a focal, firm, tan, irregular mass that is adhered to the regional dura and measures 2.3 x 3 x 3.3 cm. The regional cerebral gyri are expanded (edema). Impression smears are obtained.
The fixed brain is sectioned. In the right frontal lobe, there is an irregular, firm, tan mass with a midline shift and regional parenchymal expansion.
Laboratory Results:
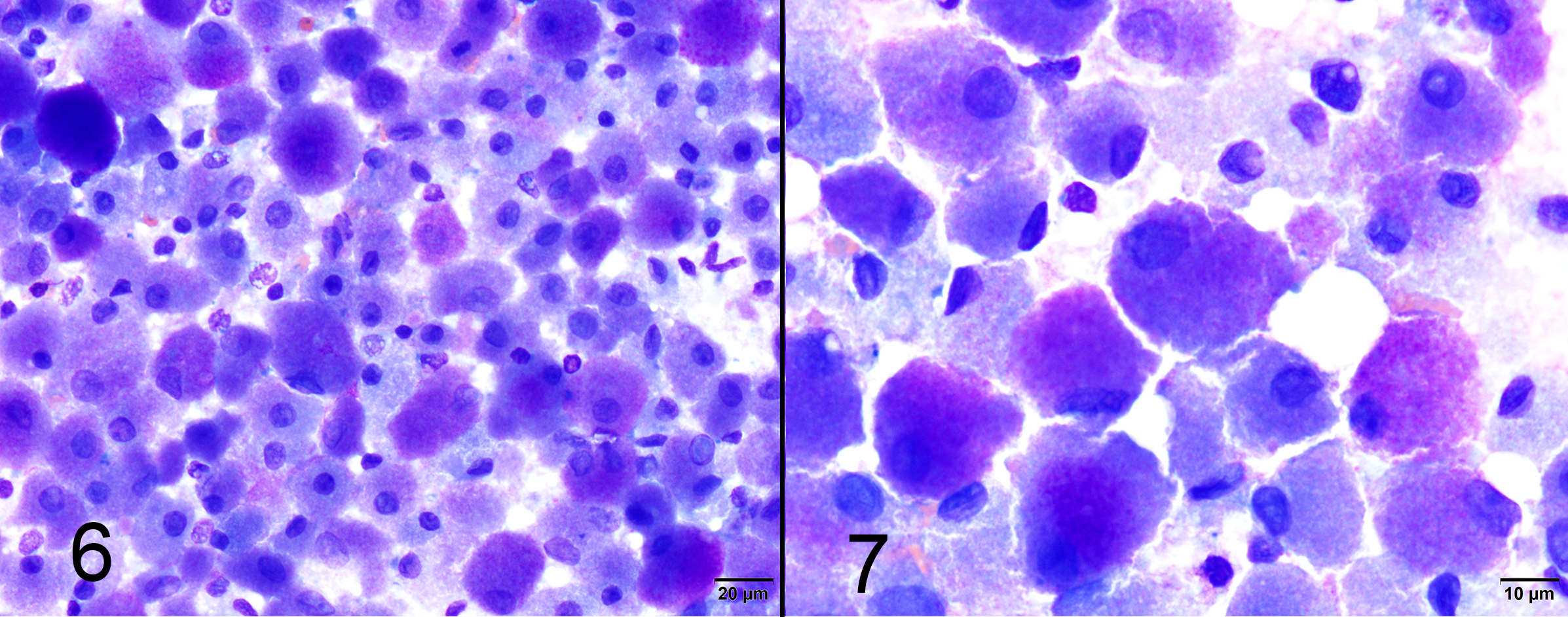
Impression smear cytology of brain mass: Smears are highly cellular and contain large numbers of round, oval and polygonal cells with abundant cytoplasm and well-defined cell borders. Cells contain large amounts of finely granulated, eosinophilic to amphophilic or basophilic cytoplasm. Nuclei are frequently eccentrically placed, round to oval and monomorphic with single or indistinct nucleoli. There is marked anisocytosis and mild anisokaryosis.
Microscopic Description:
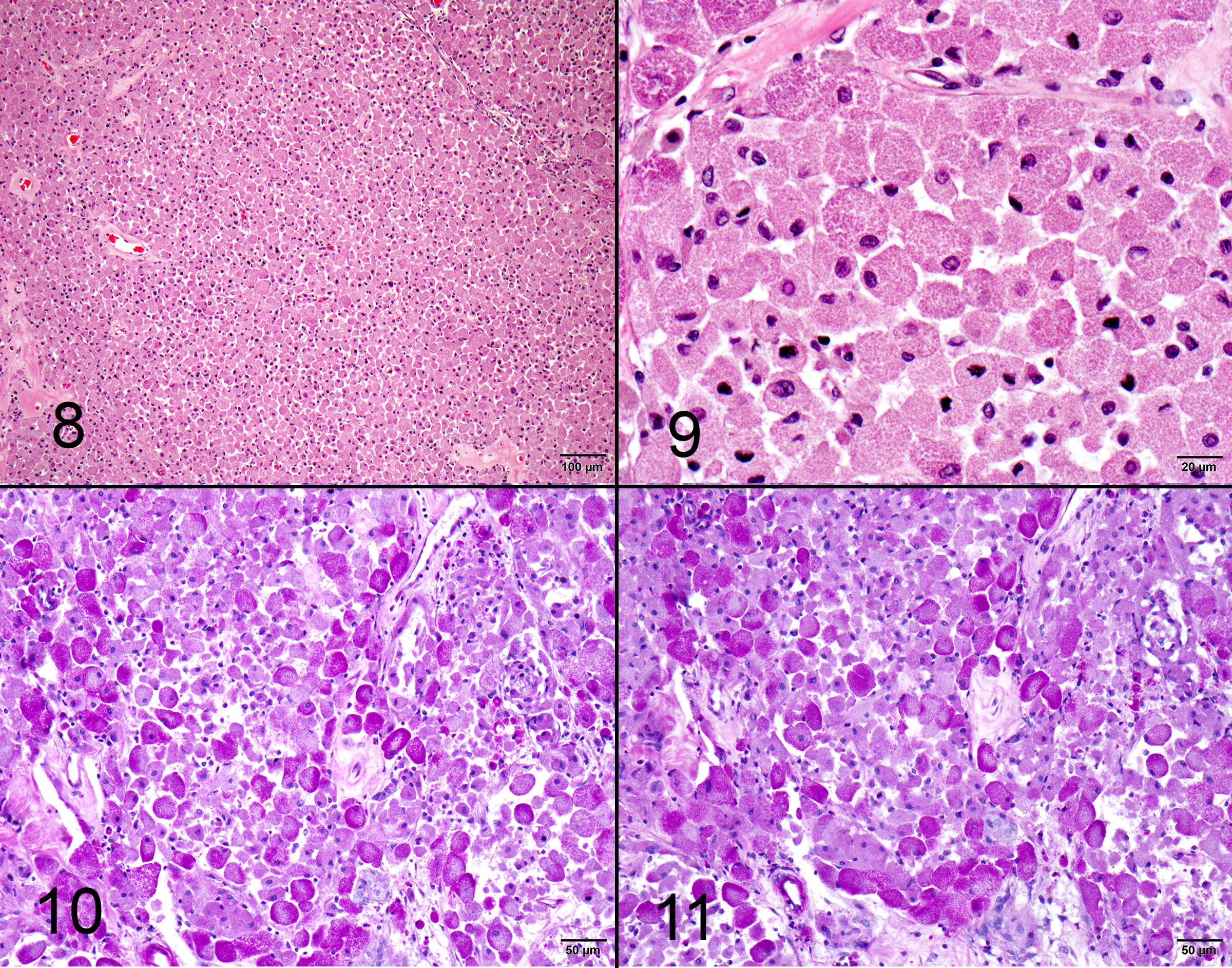
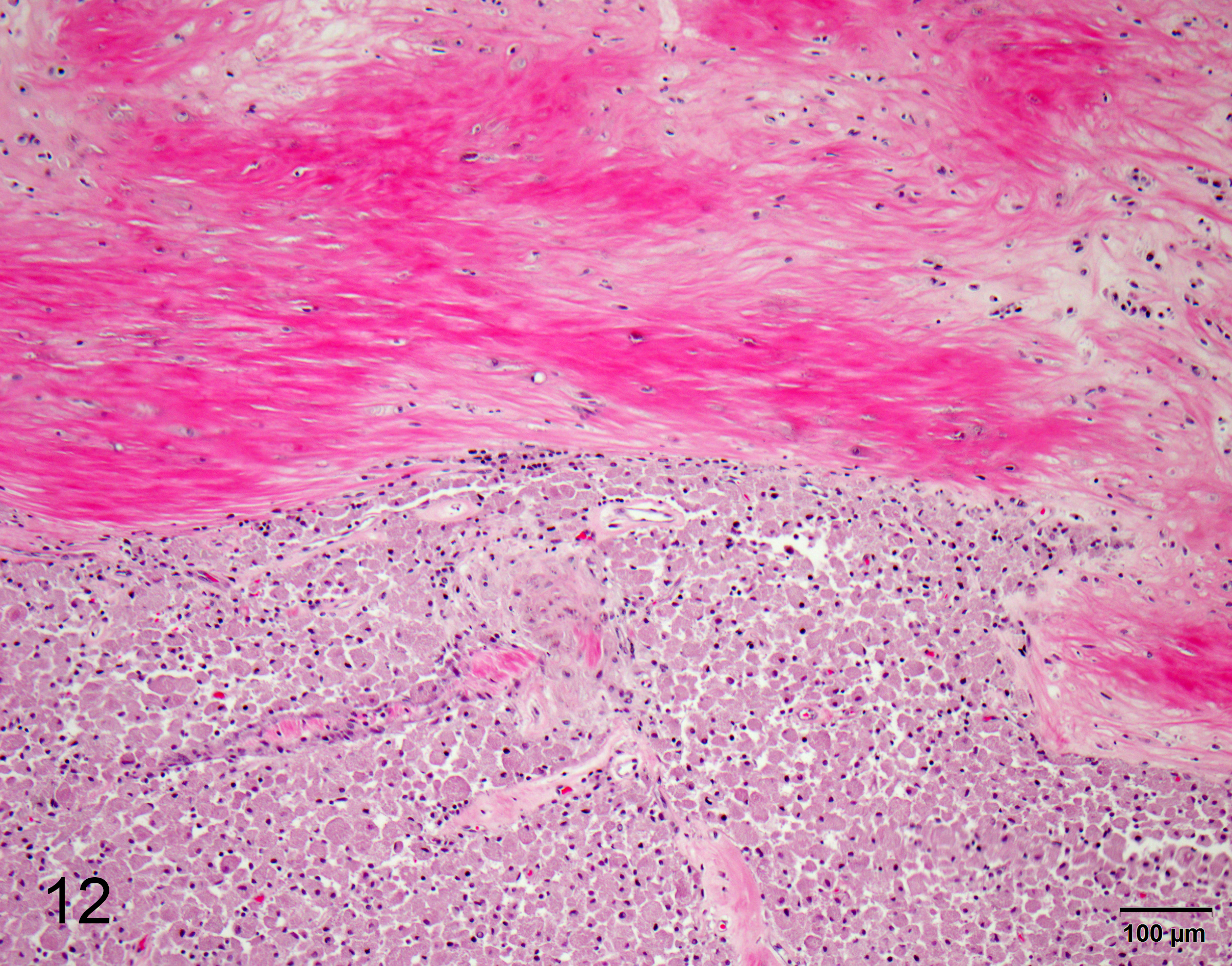
One section of rostral cerebrum is examined. Extending from and expanding the leptomeninges, infiltrating into the regional cerebral cortex, there is a plaque-like, irregular, unencapsulated, mildly infiltrative neoplasm measuring approximately 1.8 x 1.0 cm. The neoplasm is comprised of large round, oval and polygonal cells forming sheets and supported by a scant, fibrovascular stroma. Neoplastic cells have well defined cell borders and contain abundant cytoplasm with densely packed eosinophilic granules. Nuclei are frequently eccentric, round, oval and sometimes angular, exhibiting mild pleomorphism (up to 2-fold) with finely stippled chromatin and an indistinct or single central nucleolus. There are zero mitoses in ten 40x (2.37 mm2) high power fields. The leptomeninges within and around the tumor undergoes fibrosis, with multifocal regions of chondroid metaplasia. The regional infiltrated cortex exhibits parenchymal rarefaction, vacuolation, increased numbers of small caliber vessels lined by hypertrophied endothelial cells, increased numbers of glial cells comprised of microglial (including Gitter cells) and astrocytes. Multifocally, dilated, eosinophilic axons are observed (spheroids). Occasional foci of karyorrhectic debris, amorphous eosinophilic material and hemorrhage are present (necrosis). Rare perivascular aggregates of inflammatory cells are observed throughout the parenchyma and leptomeninges, comprised of lymphocytes, plasma cells and fewer macrophages containing golden brown, granular pigment (hemosiderin). Small vessels are infrequently mineralized.
Contributor’s Morphologic Diagnoses:
Brain (rostral cerebrum): Granular cell tumor with regional infiltration, edema, gliosis, and leptomeningeal fibrosis with chondroid metaplasia
Special stains: Cytoplasmic granules are PAS positive and diastase resistant
Contributor’s Comment:
Granular cell tumors (GCTs) are most common in the meninges of the cerebrum in dogs.2 In dogs, these tumors are typically supratentorial and extra-axial, plaque-like and infiltrative, however, can also cause diffuse thickening of the meninges.2 GCTs are typically unilateral, which can result in midline shifts caused by cerebral edema (as in this case). There are few reports of GCTs in the peripheral nervous system of dogs.4 These tumors are characteristically comprised of large cells with abundant, granular eosinophilic cytoplasm.2 The granular appearance of the cytoplasm is thought to be due to the presence of abundant lysosomes.2 The cell of origin is unknown and is disputed in the literature.2,5 It has been suggested that GCTs represent a common phenotype expressed by a variety of tumor cells.1,3 Tumors that can exhibit granular changes are many and include meningiomas and other primary tumors of the CNS, paragangliomas, schwannomas, neurofibromas, leiomyomas, hibernomas, fibroxanthomas and rhabdomyomas.4 In humans, peripheral GCTs are of Schwann cell origin, while intracranial GCTs are derived from astrocytes and pituicytes,5 commonly occurring within the infundibulum or neurohypophysis.3 Granular cells are frequently observed in other types of primary brain tumors,2 which may reflect a nonspecific metabolic transformation.3 A granular cell component has been reported in some human astrocytomas, meningiomas, and oligodendrogliomas.3,5
The imaging appearance of this tumor is characterized by strong, uniform, contrast enhancement and T1 weighted hyperintensity, as observed in this case.2 The presence of T1 weighted hyperintensity in GCTs is considered a useful diagnostic feature.2 These tumors can also be hyperintense on T2 weighted and FLAIR images,1,2 however, this mass was predominantly T2 isointense to faintly hypointense with patchy FLAIR hyperintensities. Additional useful imaging features include a plaque-like distribution pattern, peritumoral edema with a mass effect, meningeal involvement and an absence of bone involvement.1 These characteristics are not unique to the GCT, thus must be interpreted with caution, as GCT represents an extremely rare neoplasm of the canine central nervous system.1
Granular cell tumors typically stain strongly with PAS and are diastase resistant.1-5 With immunohistochemistry, the granules are immunoreactive for ubiquitin,2,3 with variable immunoreactivity for Vimentin, S-100, α 1-antichymotrypsin and α 1-antitrypsin.2,3 GCTs are consistently negative for GFAP, pancytokeratins, leukocyte and macrophage markers.3 The uniform positive ubiquitin staining may indicate cytosolic proteosome degradation activated by ubiquitin dependent processes or breakdown of proteins facilitated by protein-ubiquitin conjugates mediated by the endosome-lysosome system.3 Staining of granules with anti-proteinases (α-1 antitrypsin and chymotrypsin) was initially thought to reflect a possible histiocyte origin, however, these findings are of limited specificity and histiocytic origin is not considered likely.3 Ultrastructural evaluation reveals that the granular cytoplasm contains densely packed autophagosomes containing residual and dense bodies, irregular and membrane bound granules, multivesicular bodies, empty vesicles and membrane-like whorls.2,3 Lysosomal accumulation is thought to be secondary to cellular degeneration, lysosomal enzyme defects and autophagy.5 Schwann or meningeal cell origin is considered less likely due to the lack of desmosomal or gap junctions as well as peripheral basal lamina.2
Fibrosis was prominent in the regional leptomeninges in this case, and the presence of abundant collagenous tissue has been previously reported in canine GCTs.3 In one case series, spindle shaped cells entrapped within collagenous tissue were hypothesized to transition to the large granular cells outside of the collagenous extracellular matrix.3 GCTs grow slowly and rarely metastasize,4 and in this case, the tumor was documented to be present for a period of 2 years following radiation therapy. These tumors are described in numerous other species, including people, horses, rats, cats, ferrets and birds.5 Common locations include the lungs in horses, the tongue in dogs and the meninges in rats.5 In rats, GCTs are the most common primary CNS tumor.1,3,4 Ultrastructural and morphological evidence in rats suggests that intracranial GCTs originate from arachnoid cells of the meninges.1,3
Contributing Institution:
Animal Medical Center, 510 East 62nd St. New York, NY 10065.
http://www.amcny.org
JPC Diagnosis:
Leptomeninges and cerebrum: Granular cell tumor.
JPC Comment:
There are a few recent publications on this uncommon neoplasm in novel species. This week’s moderator, MAJ Daniel Finnegan, described a meningeal granular cell tumor in a green tree python.2 Reifinger et al. documented another case of granular cell tumor in the abdominal cavity of a snake.6 These cases both had PAS positive and diastase resistant cytoplasmic granules, confirmed to be phagolysosomes on electron microscopy. These were the first two documented granular cell tumors in snakes, and granular cell tumors are rarely reported in non-mammalian species.
Granular cell tumors are characteristically compressive and non-infiltrative neoplasms. This case is unique in its infiltrative growth pattern; the moderator and participants discussed that this might be due to the chronicity or size of the neoplasm.
The contributor described the common presentations of granular cell tumors in mammalian species. A recent study demonstrated the propensity for rabbits to develop testicular granular cell tumors.7 In 52 rabbits with testicular tumors, 63% were granular cell tumors, and 11 of 36 had bilateral granular cell tumors.7 While other studies cite interstitial cell tumors as the most common testicular tumor in rabbits, this study suggests that granular cell tumors may be more common.
References:
- Anwer, C.C., Vernau, K.M., Higgins, R.J., et al. Magnetic resonance imaging features of intracranial granular cell tumors in six dogs. Vet Radiol Ultrasound 2013; 54:271-277.
- Finnegan DK, Cartoceti AN, Hauck AM, LaDouceur EEB. Meningeal Granular Cell Tumor in a Green Tree Python (Morelia viridis). J Comp Path. 2020; 174: 54-57.
- Higgins RJ, Bollen AW, Dickinson PJ, Siso-Llonch, S. Tumors of the Nervous System. In: Tumors in Domestic Animals, 5th Ames, IA: John Wiley & sons, Inc; 2017:870-872.
- Higgins, R.J., LeCouteur, R.A., Vernau K.M., et al. Granular cell tumors of the canine central nervous system: two cases. Vet Pathol 2001; 38:620-627.
- Rao, D., Rylander, H., Drees, R., et al. Granular cell tumor in a lumbar spinal nerve of a dog. J Vet Diagn Invest 2010; 22:638-642.
- Reifinger M, Dinhopl N, Gumpengberger M, Konecny M, Cigler P. Granular Cell Tumour in a California Kingsnake. J Comp Path. 2020; 175: 24-28.
- Reineking W, Seehusen F, Lehmbecker A, Wohlsein P. Predominance of Granular Cell Tumors among Testicular Tumours of Rabbits (Oryctolagus cuniculi dom.). J Comp Path. 2019; 153: 24-29.
- Spoor MS, Kim DY, Kanazono S. et al. What is your diagnosis? Impression smears of a cerebral mass from a dog. Vet Clin Pathol 2013; 42(2):240-241.