CASE 3: PV200 (4119010-00)
Signalment:
A 4.5 years old, male castrated, Yorkshire terrier (Canis familiaris)
History:
Vomiting. Mass observed in the iliocecal junction on ultrasound. Resection and anastomosis of a distal portion of ileum and cecum with a mass on exploratory abdominal surgery.
Gross Pathology:
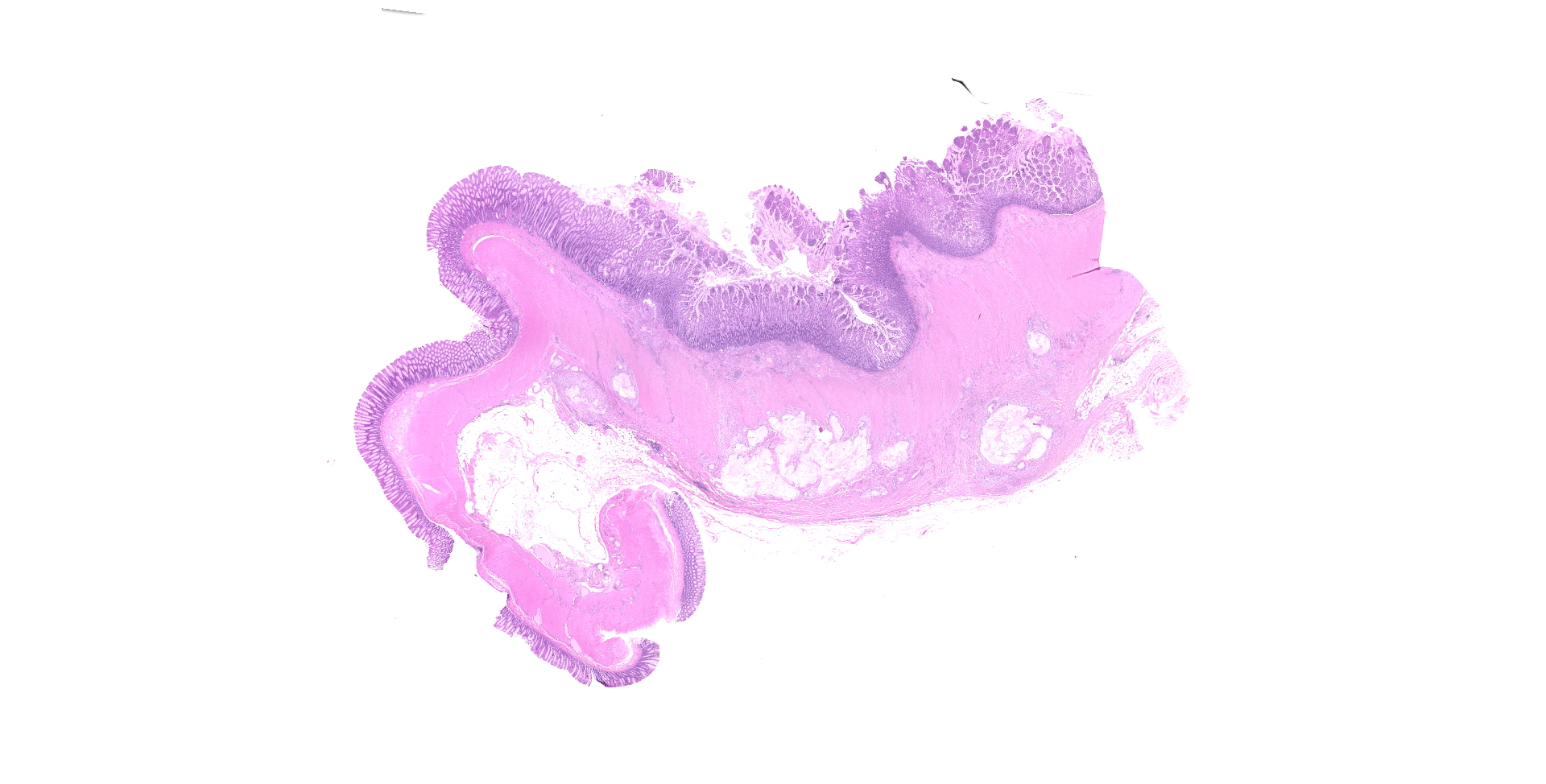
On exploratory surgery, a mass in the intestinal wall, at the ileocecal junction.
Laboratory results:
Normal serum protein.
Microscopic description:
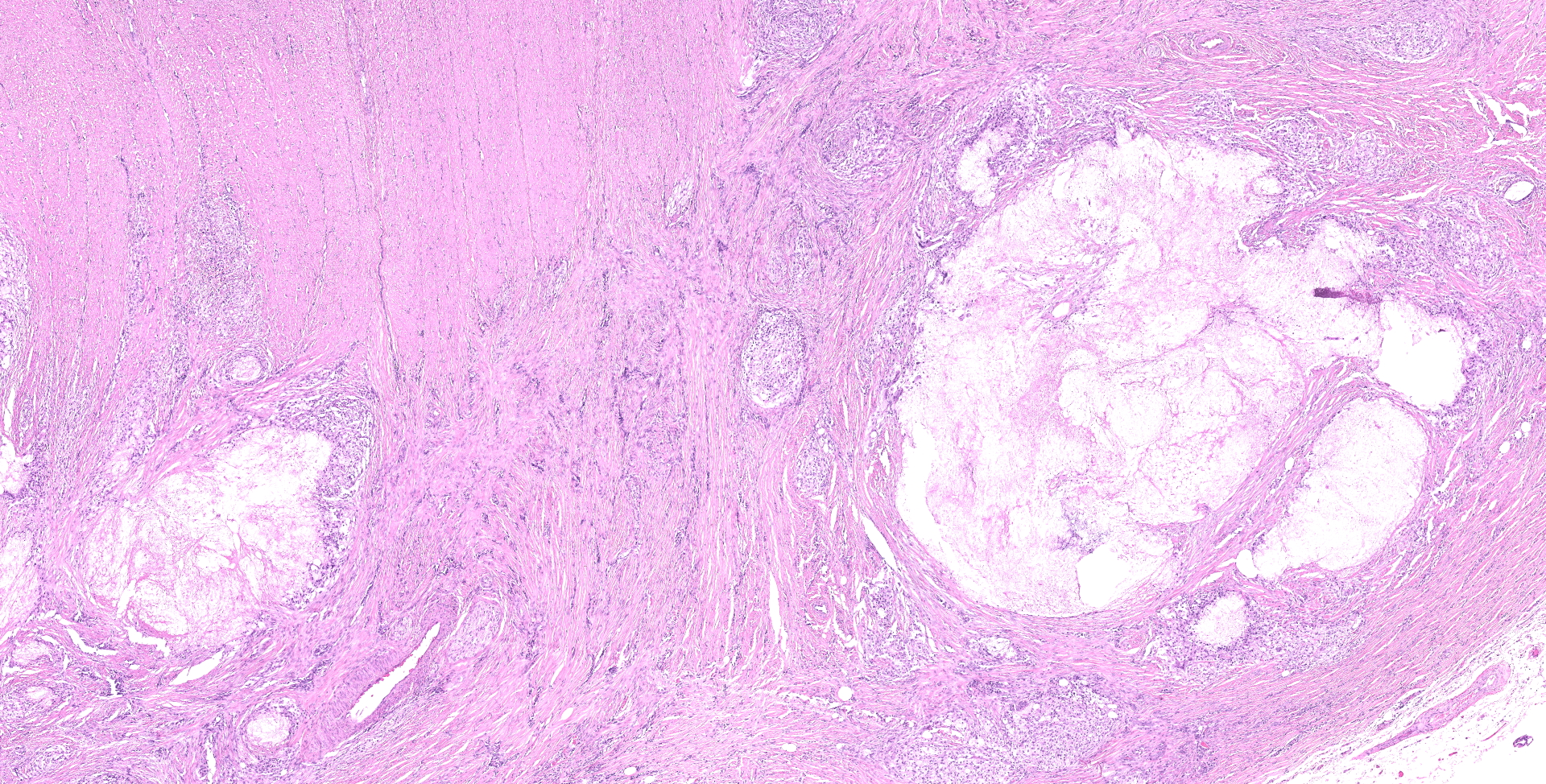
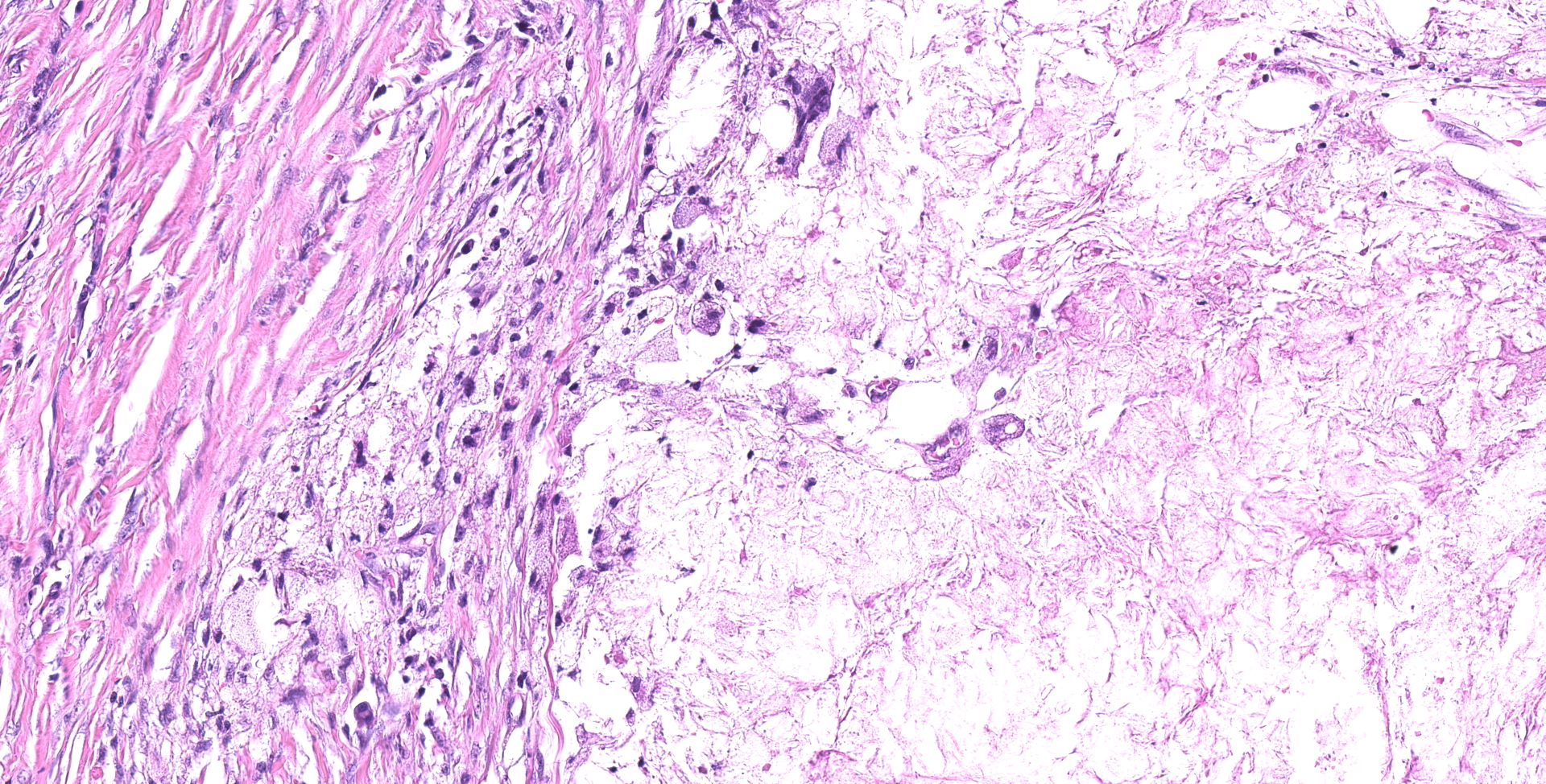
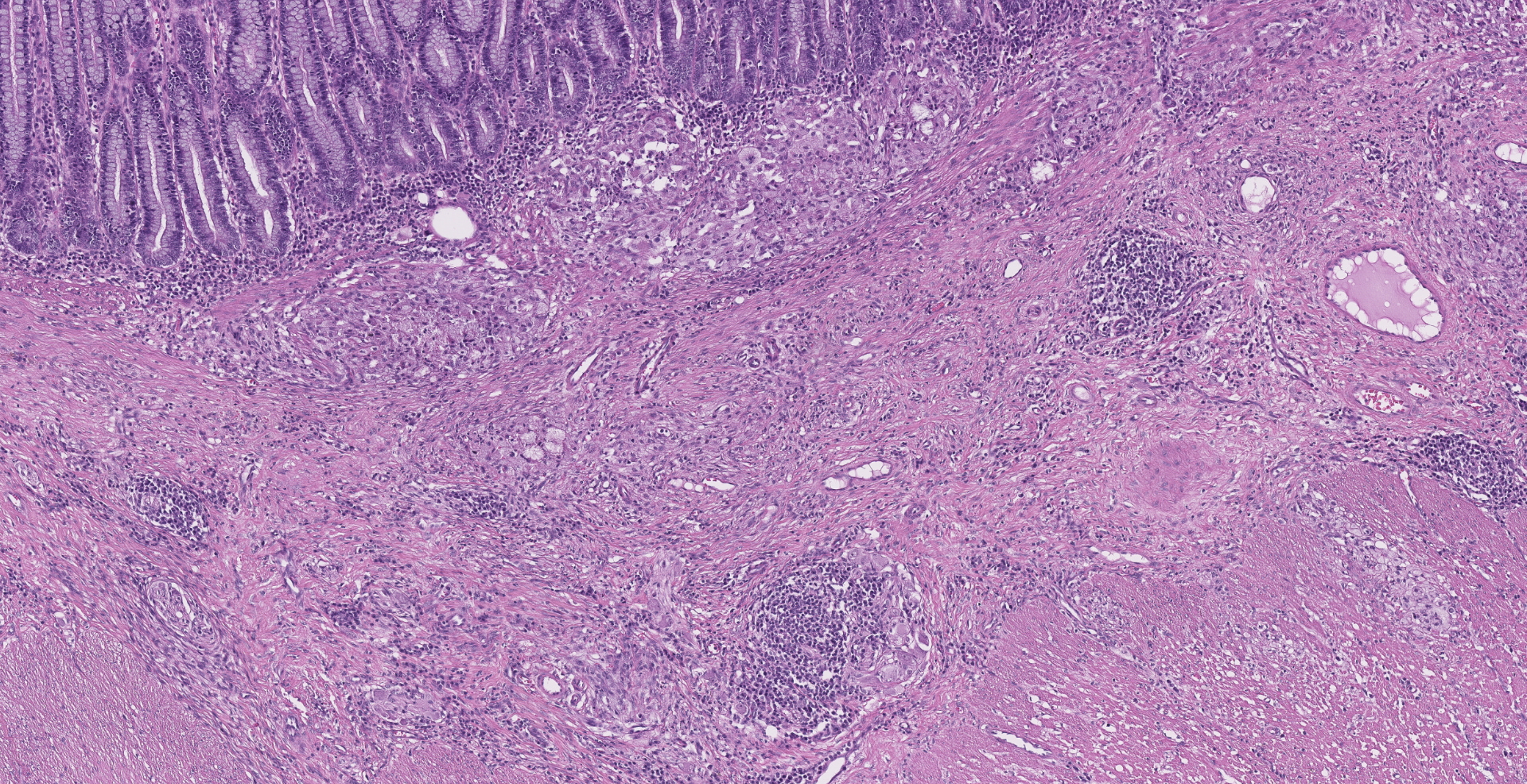
Ileocecocolic junction. There are multifocal areas of saponification and cholesterol clefts present along the serosal margin, in the tunica muscularis and occasionally in the submucosa. These areas are surrounded by macrophages and multinucleated giant cells, with peripheral reactive fibrous tissue (granulomas). There is mild dilation of lymphatics in the submucosa and there is rare lymphangiectasia in the lamina propria.
Contributor's morphologic diagnosis:
Focal mural intestinal lipogranulomatous lymphangitis
Contributor's comment:
Lipogranulomatous lymphangitis is inflammation of the intestine and lymphatic vessels and surrounding tissues caused by chronic leakage of lipid-laden chyle.6 The most common cause of this type of finding is lymphangiectasia with protein losing enteropathy. The finding is usually diffuse along the serosa and mesentery of the small intestine.4,6
Lymphangiectasia has been described in the dog as one of the most common cause of malabsorption/protein losing enteropathy.4 Dilated intestinal lacteals result in lymph leakage into the small bowel lumen with subsequent protein-losing enteropathy with chronic diarrhea, wasting, hypoproteinemia, lymphopenia, hypercalcemia and hypocholesterolemia. Lymphangiectasia is associated with a syndrome that includes peripheral edema, ascites and hydrothorax, the result of hypoalbuminemia.4
Breed predisposition includes Yorkshire terriers and Norwegian Ludenhunds, in which lymphangiectasia is part of the syndrome of protein losing enteropathy with inflammatory bowel disease and in which gastritis in gastric neoplasia may be concomitant.4,6
In this case however, the dog had normal serum protein and the lesion was restricted to the iliocecal junction. There are two relatively recent reports2,5, of dogs with distal small intestinal thickening or masses, with evidence of lipogranulomatous lymphangitis. These dogs had normal total protein, albumin, globulin and cholesterol concentrations. The dogs presented with diarrhea, vomiting, abdominal pain and weight loss.
Abdominal ultrasound indicated thickening of the distal small intestine or a mural mass in the ileocecal junction. There was no evidence of diffuse lymphangiectasia.
The histologic lesions were similar in all dogs. The lesions were in the distal small intestine. The wall of the intestine was expanded by inflammation, dilated lymphatics and fibrosis. There was formation of granulomas with central saponified fat or cholesterol clefts surrounded by neutrophils, foamy macrophages and multinucleated giant cells.
Postoperative outcomes ranged from remission of clinical signs without treatment for 10 to 12 months in two dogs, postoperative management with medical and nutritional management in three dogs and no outcome for one case.
Contributing Institution:
Department of Veterinary Resources
Weizmann Institute
Rehovot 76100, Israel
http://www.weizmann.ac.il/vet/
JPC diagnosis:
Ileum, cecum: Lymphangitis, lipogranulomatous, diffuse, chronic, severe, with mild multifocal lymphangiectasia, Yorkshire terrier, canine.
JPC comment:
The contributor provides a concise summary of lipogranulomatous lymphangitis in the dog. As they often are found concurrently, a short overview of lymphangiectasia is now provided. There are reported cases of both primary and focal canine lymphangiectasia, the latter being discussed by the contributor.
While there are tools and algorithms for standardized scoring of endoscopic biopsies of stomach, duodenum, and the colon from dogs and cats, they capture only one dimension of lymphatic characterization. The WSAVA guidelines classifies villous lacteal width into normal (up to 25% width of villous lamina propria), mildly dilated (up to 50% width), moderate dilation (up to 75% width), and marked lacteal dilation (up to 100% of villous lamina propria).1 Chronic inflammatory enteropathy (CIE) is applied to gastrointestinal tract conditions that persist for at least 3 weeks' duration, under which intestinal lymphangiectasia may be classified. In addition to villous lacteal dilation, non-villous mucosal lacteal dilation data may help increase classification of disease or inform prognosis.7 Recent research has shown there may be a statistically significant correlation between both villous and non-villous lacteal widths in the ileum and dogs that have CIE with protein losing enteropathy. Unfortunately, while WSAVA recognizes important and relevant information that can be gained from ileal biopsies, there is not yet a standardized template for evaluation.8
Lymphatic endothelium is derived from venous progenitor cells and express certain antigens that distinguish them from blood vessel endothelium. Depending on availability, useful immunohistochemical stains include the nuclear transcription factor human prospero homeobox (Prox-1), lymphatic vascular endothelial hyaluronic acid receptor (LYVE-1), and podoplanin (D2-40).7
Dogs (and other animals) with intestinal lymphangiectasia often have derangements of nutritional absorption that is clinically measurable, including hypoproteinemia, hypogammaglobulinemia, and lymphopenia. It has been found that dogs with intestinal lymphangiectasia have fewer total plasma cells in the orad portions of the intestine, and higher plasma cell concentrations in the aborad sections. While changes in distribution of plasma cells, as well as the distribution of IgA, IgM, and IgG secreted are apparent, the significance remained undetermined.3
References:
1. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Path. 2008;138:S1-S43.
2. Lecoindre A et al. Focal intestinal Lipogranulomatous lymphangitis in 10 dogs. J Small Anim Practice, 2016, 57(9):465-71.
3. Suter MM, Palmer DG, Schenk H. Primary intestinal lymphangiectasia in three dogs: A morphological and immunopathological investigation. Vet Pathol. 1985;22:123-130.
4. Uzal FA et al. Alimentary system. In: Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Ed: M Grant Maxie. 6th ed. vol. 2, Elsevier, 2016:90.
5. Van Kruinigen HJ, et al. Lipogranulomatous Lymphangitis in Canine Intestinal Lymphangiectasia. Vet. Pathol, 1984, 21: 377-383.
6. Watson VE, et al. Focal Intestinal Lipogranulomatous Lymphangitis in 6 Dogs (2008?2011). J Vet Intern Med, 2014; 28:48?51.
7. Wennogle SA, Priestnall SL, Suarez-Bonnet A, Soontararak S, Webb CB. Lymphatic endothelial cell immunohistochemical markers for evaluation of the intestinal lymphatic vasculature in dogs with chronic inflammatory enteropathy. Journal of Veterinary Internal Medicine. 2019;33:1669-1676.
8. WSAVA International Gastrointestinal Standardization Group. Endoscopic, Biopsy, and Histopathologic Guidelines for the Evaluation of Gastrointestinal Inflammation in Companion Animals. J Vet Intern Med. 2010;24:10-26.