CASE 3: 2019 Case 2 (4135953-00)
Signalment: Intact, female, 24-year-old, patas monkey (Erythrocebus patas)
History: This monkey presented with bloat prior to humane euthanasia.
Gross Pathology:
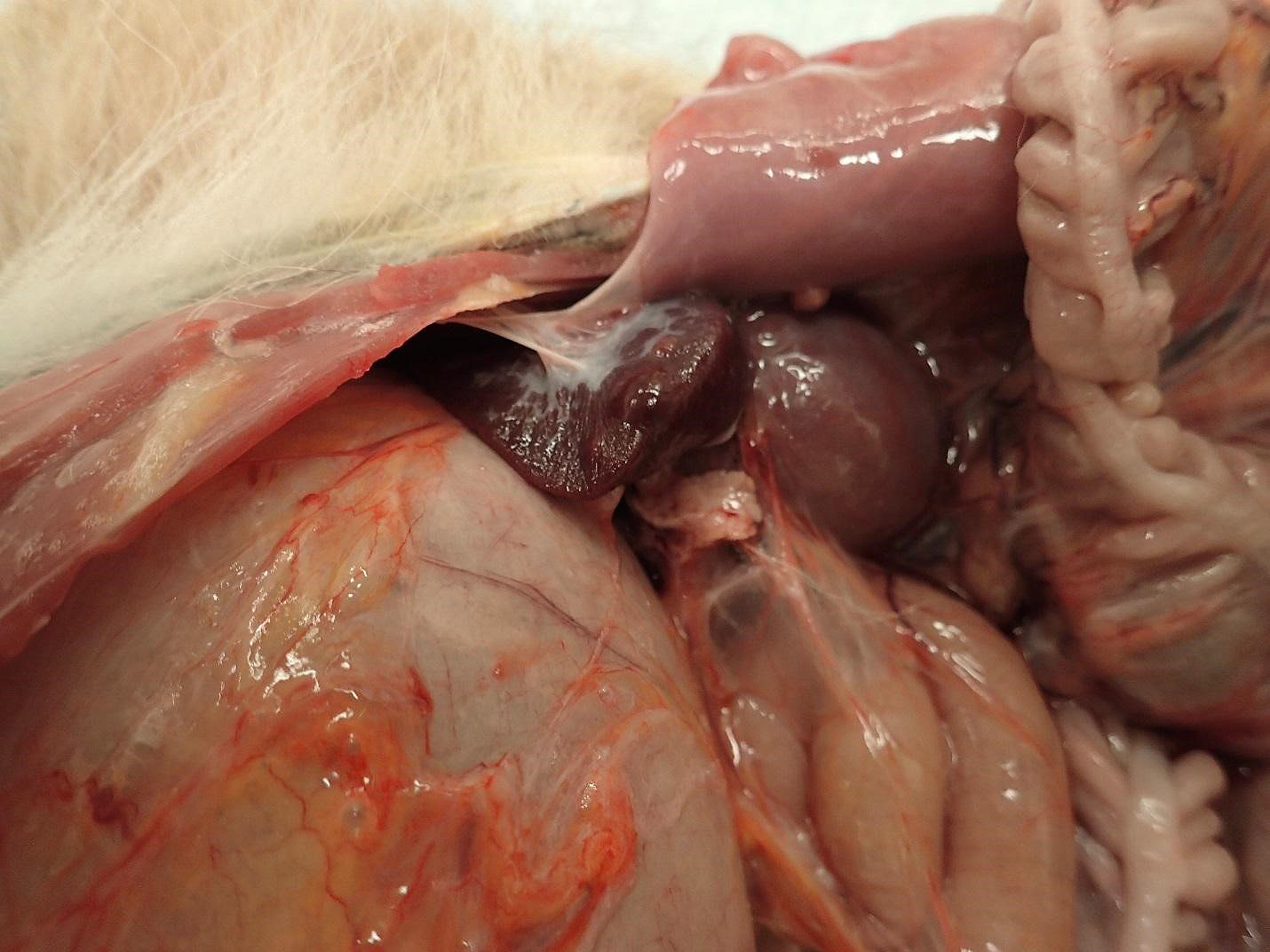
Spleen:
1. Chronic, focal, moderate fibrous capsular adhesion to the body wall
2. Multifocal, firm, 0.3-0.8 cm, dome-shaped, raised nodules
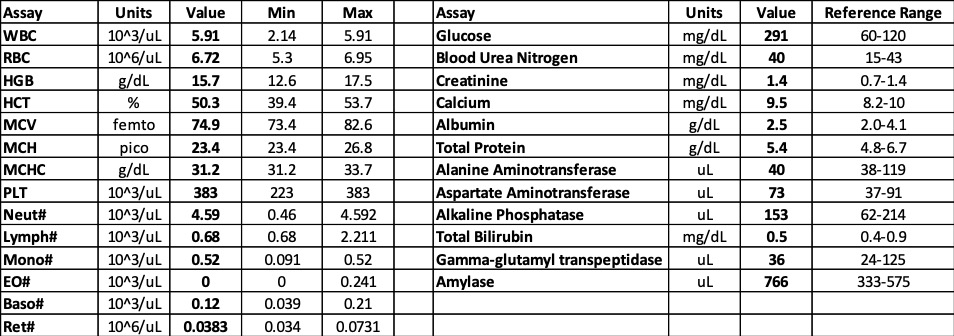
Laboratory results:
See table.
Microscopic description:
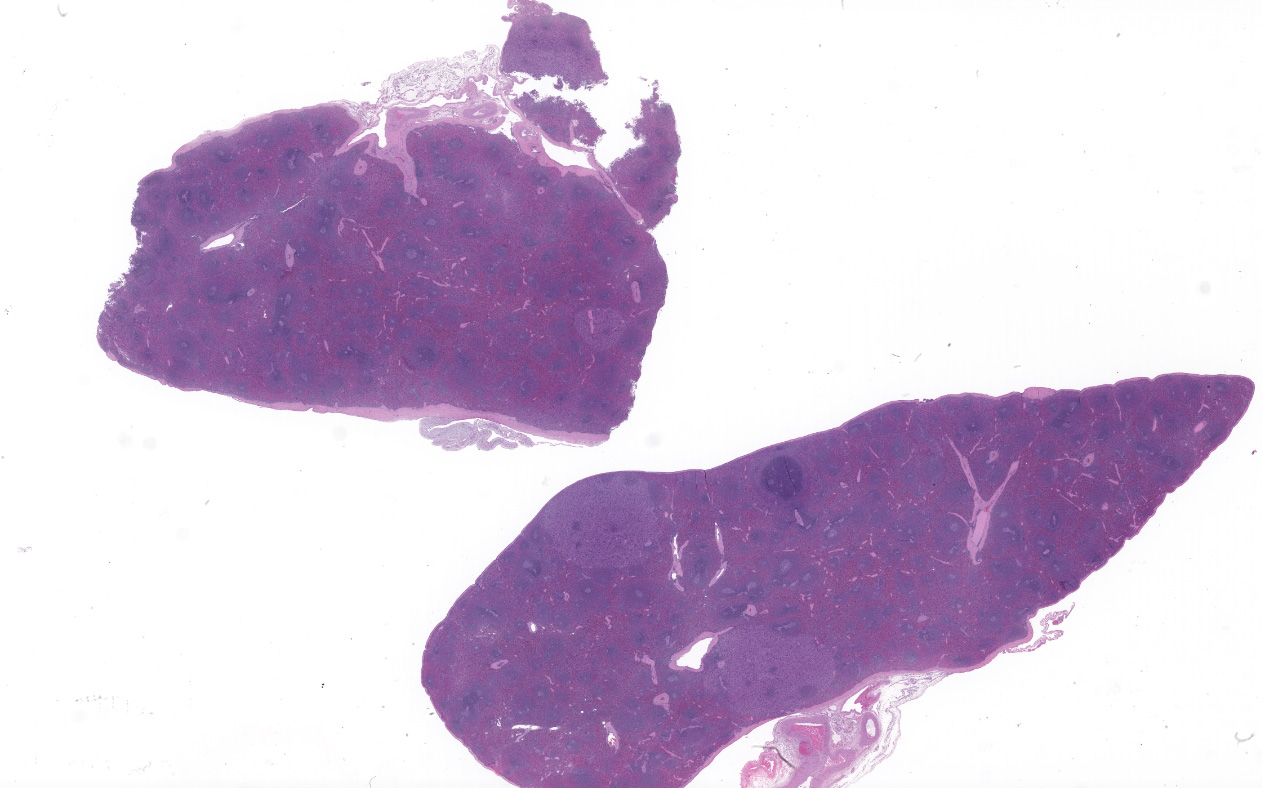
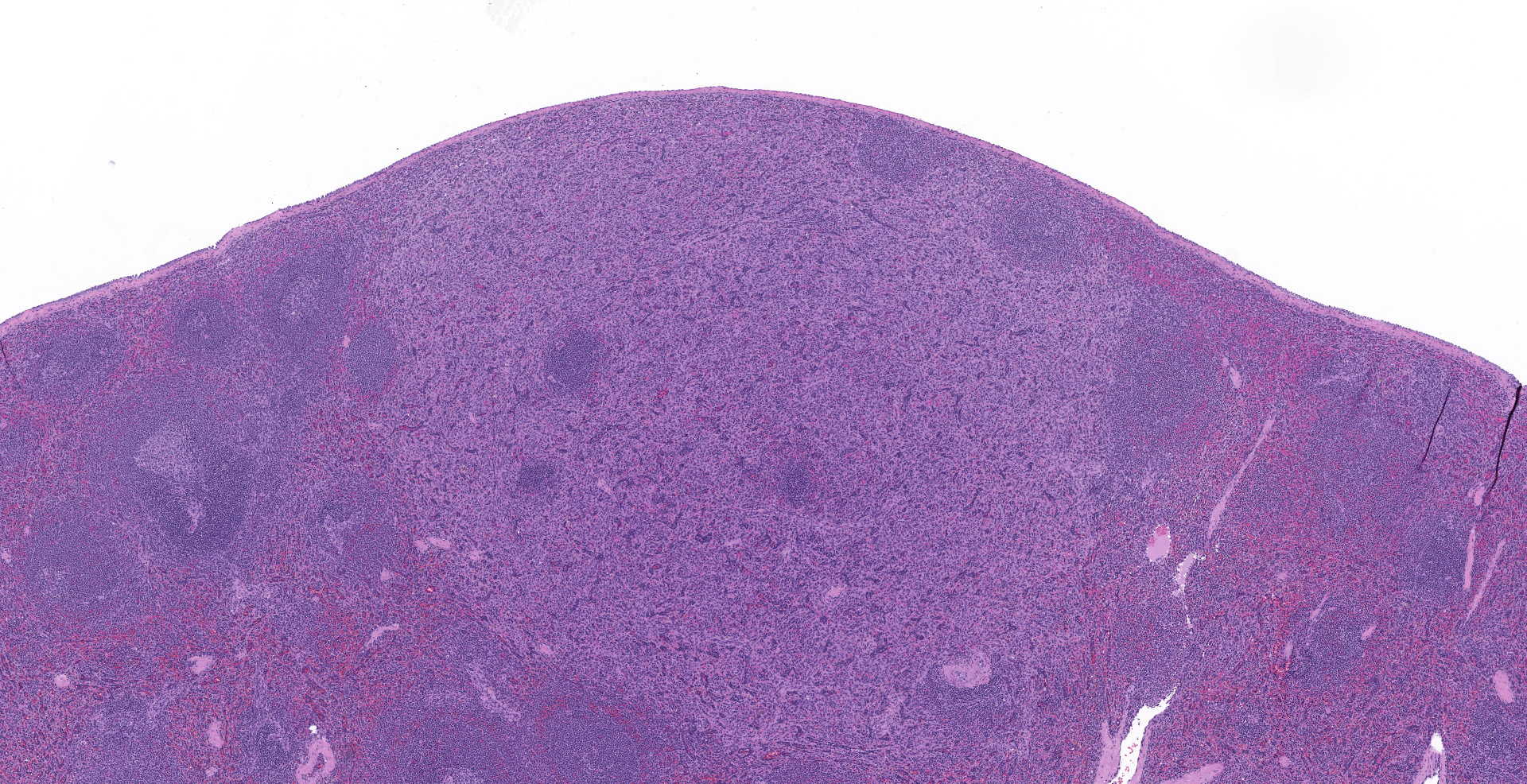
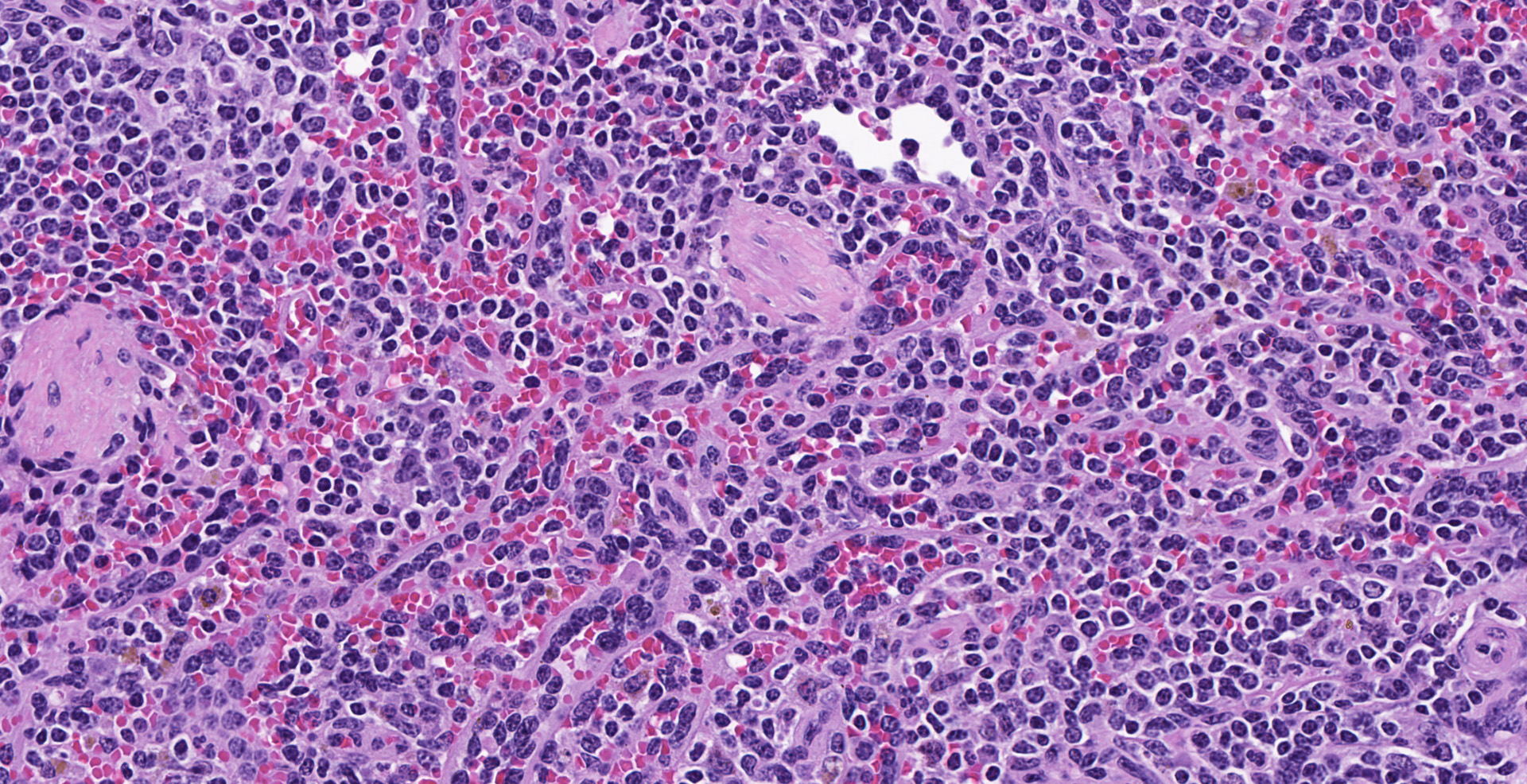
Spleen (2 sections): Multifocally, the splenic parenchyma contains several nodular aggregates
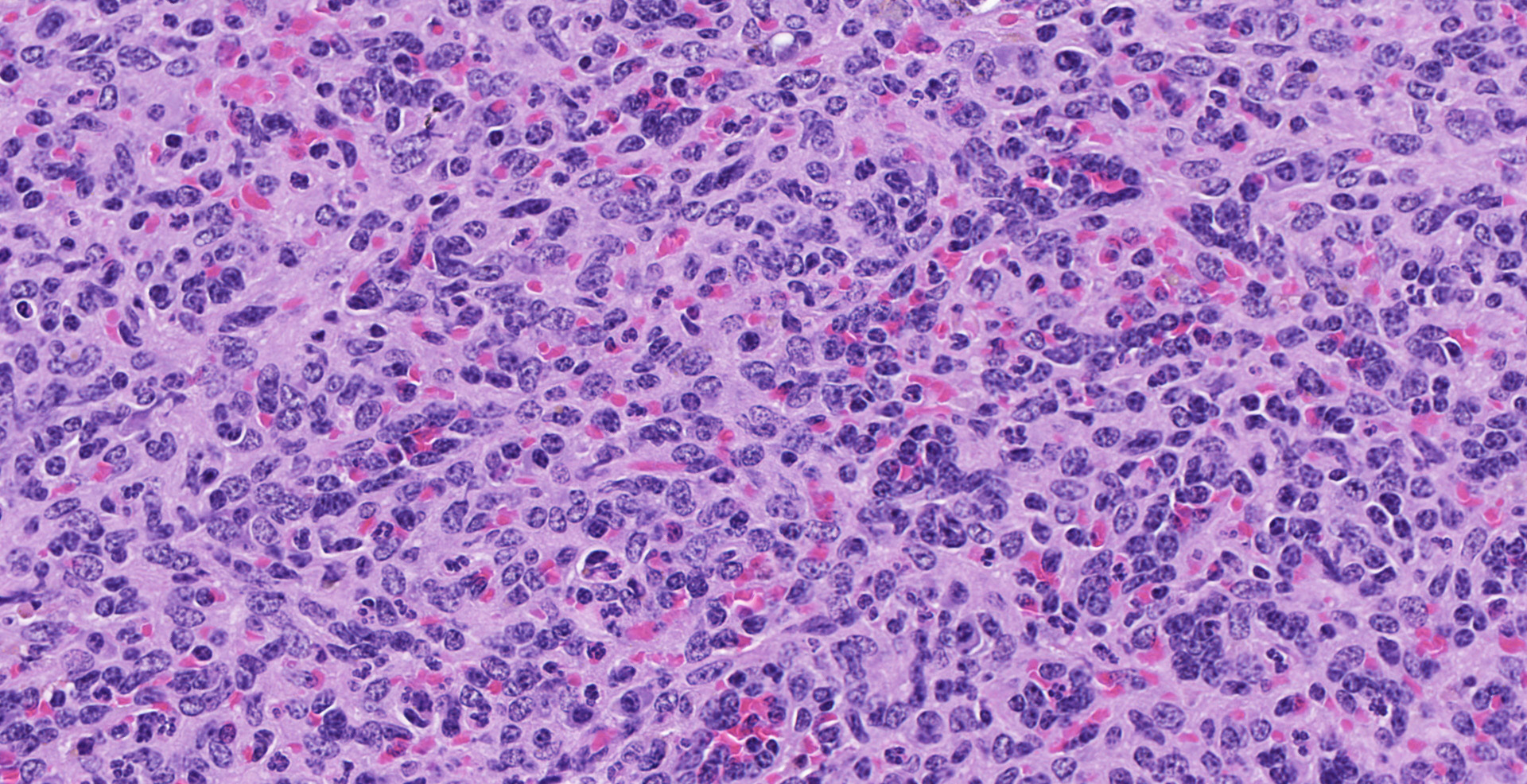
of polygonal to elongated mesenchymal cells forming variably sized, vascular channels separated by scant to moderate amounts of eosinophilic stroma. The cells lining the vascular channels are plump, contain open-faced, oval to elongated nuclei containing one to four nucleoli and scant eosinophilic cytoplasm. Anisocytosis and anisokaryosis are minimal. The vascular channels formed by this mesenchymal cell population are minimally to widely separated by a bland, eosinophilic stroma that largely replaces the pre-existing red pulp in locally extensive areas. In areas where the stroma accompanying these vascular channels is abundant, these vascular channels form distinct, unencapsulated nodules. Small numbers of hemosiderophages and neutrophils infiltrate these nodules multifocally. The splenic hilar connective tissue is mildly edematous diffusely and infiltrated by small numbers of neutrophils and macrophages. A densely collagenous, well-vascularized fibrous adhesion is attached to the capsule of the spleen. The mesothelium lining the splenic capsule is plump diffusely (reactive) and within one focal area, the mesothelium is 8 cells in thickness.
Contributor's morphologic diagnosis:
Spleen:
1. Sclerosing angiomatoid nodular transformation (SANT, presumptive)
2. Chronic, locally extensive, moderate capsular fibrosis (adhesion)
Contributor's comment:
First reported as ?cord capillary hemangioma? in 19938,9, less than 150 SANT cases have been reported in the literature and the pathogenesis is poorly understood. The actual number of cases in the literature is unknown as the same lesion has been referred to by different authors as a hamartoma, hemangioma, and multinodular hemangioma. As the lesion is considered benign, differentiating between these morphologic diagnoses has questionable clinical relevance.1,6,8 SANTs are thought to be a reactive, post-inflammatory or post-thromboembolic lesion.2 Martel et al. describes the lesion as, ?altered red pulp tissue that had been entrapped by a nonneoplastic stromal proliferative process.?11
In humans, the diagnosis is most often reported in middle-aged women, discovered as an incidental finding or as the result of abdominal pain and/or splenomegaly.11 Diagnostic features of SANT are their angiomatoid sclerotic nodular histopathologic appearance and mixed vascular component. Definitive diagnosis requires immunohistochemistry.2,16 Immunohistochemically, SANT lesions recapitulate the normal composition of the red pulp and contain vessels with three immunophenotypically distinct phenotypes: 1) capillaries that are CD34 positive/CD31 positive and reported to be either CD8 positive or negative (most commonly reported to be CD8 negative), 2) venules that are CD34 negative/CD31 positive/CD8 negative, and 3) red pulp sinusoids that are CD34 negative/CD31 positive/and CD8 positive.2,12,15,16 Vessels within the lesion do not stain for D2-40 confirming they are not lymphatic in origin.8,13 Variable positivity has been reported in SANT for the TNF-receptor transmembrane glycoprotein CD302,16 but the significance of the presence or absence of this marker is not known. Most plasma cells in these lesions are IgG4 positive.2 In human cases, the cells composing the stroma stain variably for smooth muscle actin (pericytes), CD31 (endothelial cells), and CD68 (histiocytes).11
Differential diagnoses for SANT include littoral cell angioma, splenic hamartoma, splenic hemangioma, splenic hemangioendothelioma, and splenic angiosarcoma. The lesion in the spleen of this patas monkey has no dense foci of inflammation consistent with an inflammatory pseudotumor (IPT) or dense, avascular scarring (post-traumatic fibrosis) but these should be included in the differential diagnosis of multinodular splenic lesions with an extensive inflammatory or fibrous component.
SANTs lack the proliferative activity of and cytologic atypia seen in angiosarcomas. Angiosarcomas lack a nodular growth pattern, are invasive, and are positive for CD68 and CD8.4,15 Hemangioendotheliomas (spindle, polymorphous, and epithelioid types) are considered intermediate in biological behavior between hemangioma and angiosarcoma have mild to moderate atypia and generally a low mitotic index14 but share some histopathologic features with SANT and this diagnosis is favored by some for SANT lesions. Others favor a diagnosis of splenic hamartoma of the red pulp that has undergone sclerosis.11,13 Hemangioendotheliomas, in addition to having greater cytologic atypia and mitotic activity than SANT, are variably immunoreactive for CD34 and cytokeratin, and are CD21, CD8, and CD68 negative. The vessels in splenic hamartomas are composed of only sinusoids (factor VIII positive/CD31 positive/CD8 positive/type IV collagen positive/CD21 negative/CD68 negative).13 Both hemangioendotheliomas and splenic hamartomas lack the multinodular growth pattern seen in SANTs. Littoral cell (sinusoidal lining cell) angioma and splenic hemangiomas also lack the multinodular, sclerotic architectural features of SANT and are also composed of a single type of blood vessel. Littoral cell angiomas are typically CD31 positive/CD34 negative/CD8 negative/CD68 positive/CD21 positive. Splenic hemangiomas are CD31 positive/CD34 positive/CD8 negative/CD21 negative/CD68 negative. Treatment for this presumed nonneoplastic lesion, is splenectomy.13 Immunohistochemistry for CD34, CD31, CD8, CD21, D2-40, factor VIII, and CD68 are pending in this case to confirm the presumptive diagnosis of SANT.
Contributing Institution:
Integrated Research Facility
Division of Clinical Research
8200 Research Plaza
Fort Detrick, MD 21702
https://www.niaid.nih.gov/about/integrated-research-facility
JPC diagnosis:
Spleen: Vascular proliferation, diffuse, moderate, with endothelial hypertrophy and random nodular sclerosis.
JPC comment:
This succinct summary outlines the highlights of what has been learned since this was first described in 2004, and prior to that was likely diagnosed as other entities. One point that bears discussion is the nature of IgG4-related disease.
All plasma cells first secrete IgM until they receive stimulation to undergo isotype switching and produce IgA, IgE, or IgG antibodies. There are four subclasses of IgG, named in the both the order of discovery and concentrations found in circulation, from IgG1 to IgG4. While IgG4 shares more than 95% sequence homology in the constant domain with the other IgG isotypes, it differs significantly in a few amino acids in the CH2 domain. This leaves it with little affinity for complement C1q binding and for Fc receptor binding. The lack of a proline in the hinge region makes its inter-heavy chain disulfide bonds more prone to reduction, allowing heavy chains to swap between IgG4 antibodies. These are sometimes called 'half-antibodies' and allows them to function as 'bi-specific', but functionally monovalent for a given antigen. One disease involving IgG4 that is well understood is pemphigus complex, where the IgG4 antibody directly attacks desmoglein (or desmocollin) to affect pathologic changes in the epidermis.12
There is increased research interest regarding this process as IgG4 production and IgG4-positive plasma cells and histologic appearance are common threads for a variety of inflammatory diseases, including sclerosing forms of sialadenitis, pancreatitis, cholangitis, aortitis, thyroiditis, and retroperitoneal fibrosis in humans. In SANT, IgG4-positive plasma cells are a consistent finding, but whether this entity will be classified as an IgG4-related disease is still to be determined.3,7,12
SANT is a uniquely splenic lesion, though histologically similar entities exist outside of the spleen. One condition most often found in lymph nodes is called nodal angiomatosis and is a subtype of vascular transformation of sinuses with a more cellular character. While perhaps histologically similar, despite different tissues, they each have different IHC morphologies, with nodal angiomatosis typically demonstrating positive immunoreactivity to desmin, smooth muscle actin, and vimentin. Conversely, the spindloid cells of SANT are negative for smooth muscle actin.5
References:
1. Benjamin BI, Mohler DN, Sandusky WR: Hemangioma of the Spleen. JAMA Internal Medicine 1965:115(3):280-284.
2. Cafferata B, Pizzi M, D'Amico F, Mescoli C, Alaggio R: Sclerosing Angiomatoid Nodular Transformation of the spleen, focal nodular hyperplasia and hemangioma of the liver: A tale of three lesions. Pathol Res Pract 2016:212(9):855-858.
3. Cao P, Wang K, Wang C, Wang H. Sclerosing angiomatoid nodular transformation in the spleen: A case series study and literature review. Medicine. 2019;98(17):e15154.
4. Delacruz V, Jorda M, Gomez-Fernandez C, Benedetto P, Ganjei P: Fine-Needle Aspiration Diagnosis of Angiosarcoma of the Spleen: A Case Report and Review of the Literature. Archives of Pathology & Laboratory Medicine 2005:129(8):1054-1056.
5. Gelberg HB, Valentine BA. Diagnostic Exercise: Lymphadenopathy associated with a thyroid carcinoma in a dog. Veterinary Pathology. 2011; 48(2):530-534.
6. J. R: Rosai and Ackerman's Surgical Pathology. Edinburgh: Mosby, 2004.
7. Jin YW, Gao W, Li FY, Cheng NS. Is sclerosing angiomatoid nodular transformation an IgG4-associated sclerosing disease? Appl Immunohistochem Mol Morphol. 2020; 28(2):e18-e20.
8. Kahn HJ, Bailey D, Marks A: Monoclonal Antibody D2-40, a New Marker of Lymphatic Endothelium, Reacts with Kaposi's Sarcoma and a Subset of Angiosarcomas. Modern Pathology 2002:15(4):434-440.
10. Krishnan J DA, Frizzera D. Use of anti-factor VIII-related antigen (F8) and QBEN10 (CD34) antibodies helps classify the benign vascular lesions of the spleen. Mod Pathol 1993:6(94A).
11. Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B, Chan JK, Rosai J: Sclerosing angiomatoid nodular transformation (SANT): report of 25 cases of a distinctive benign splenic lesion. Am J Surg Pathol 2004:28(10):1268-1279.
12. Nirula A, Glaser SM, Kalled SL Taylora FR. What is IgG4? A review of the biology of a unique immunoglobin subtype. Current Opinion in Rheumatology. 2011;23:119-124.
13. Pradhan D, Mohanty SK: Sclerosing angiomatoid nodular transformation of the spleen. Arch Pathol Lab Med 2013:137(9):1309-1312.
14. Requena L, Kutzner H: Hemangioendothelioma. Seminars in Diagnostic Pathology 2013:30(1):29-44.
15. Wang TB, Hu BG, Liu DW, Gao ZH, Shi HP, Dong WG: Sclerosing angiomatoid nodular transformation of the spleen: A case report and literature review. Oncol Lett 2016:12(2):928-932.
16. Zhou J, Zhang D, Hu G, Zheng X, Shen Q, Li W, et al.: Upregulated expression of CD30 protein in sclerosing angiomatoid nodular transformation (SANT): studies of additional 4 cases and analyses of 6 cases previously published cases. Int J Clin Exp Pathol 2015:8(6):6064-6069.