Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 5
September 27th, 2017
CASE III: 16H0097 (JPC 4101308).
Signalment: 1 to 2-year-old, male, Central European boar (Eurasian wild pig), Sus scrofa, porcine.
History: The perished animal was detected on a hunting premise in early December. Several perished wild boars of the same age had been found during the last months.
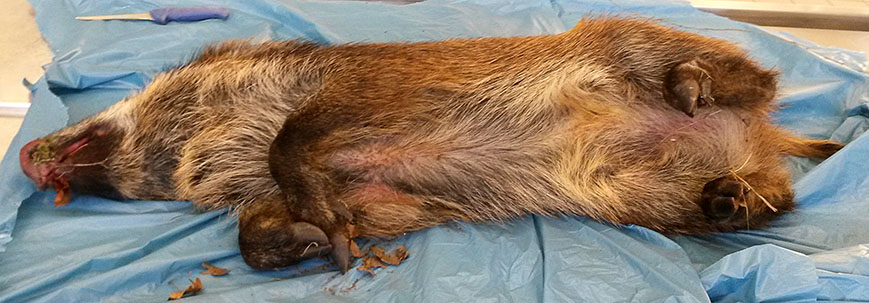
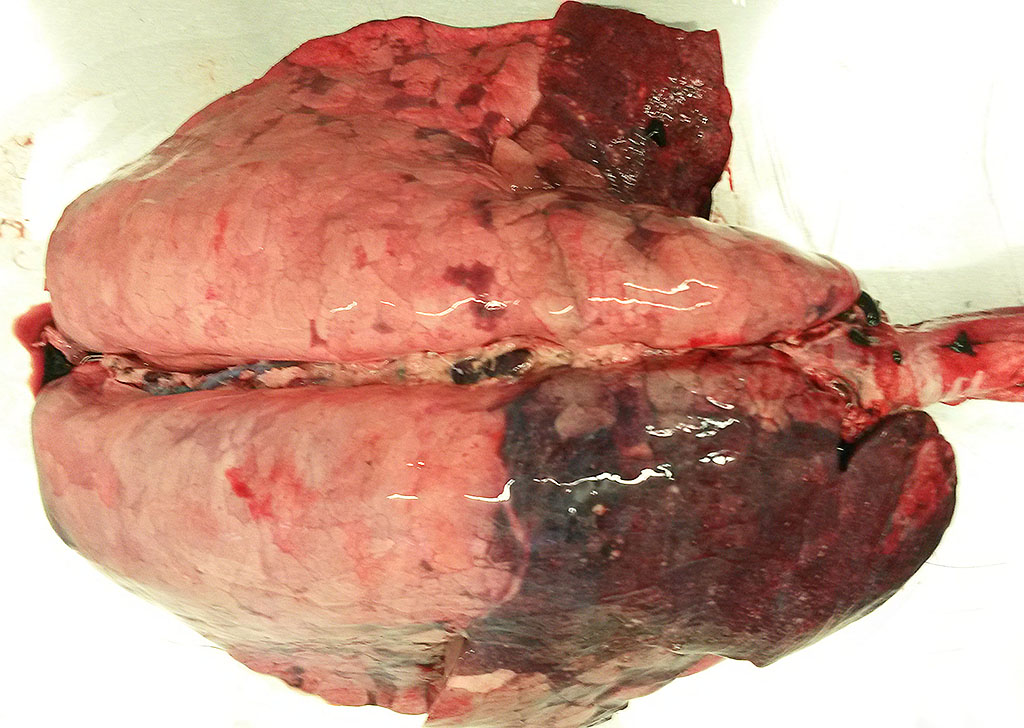
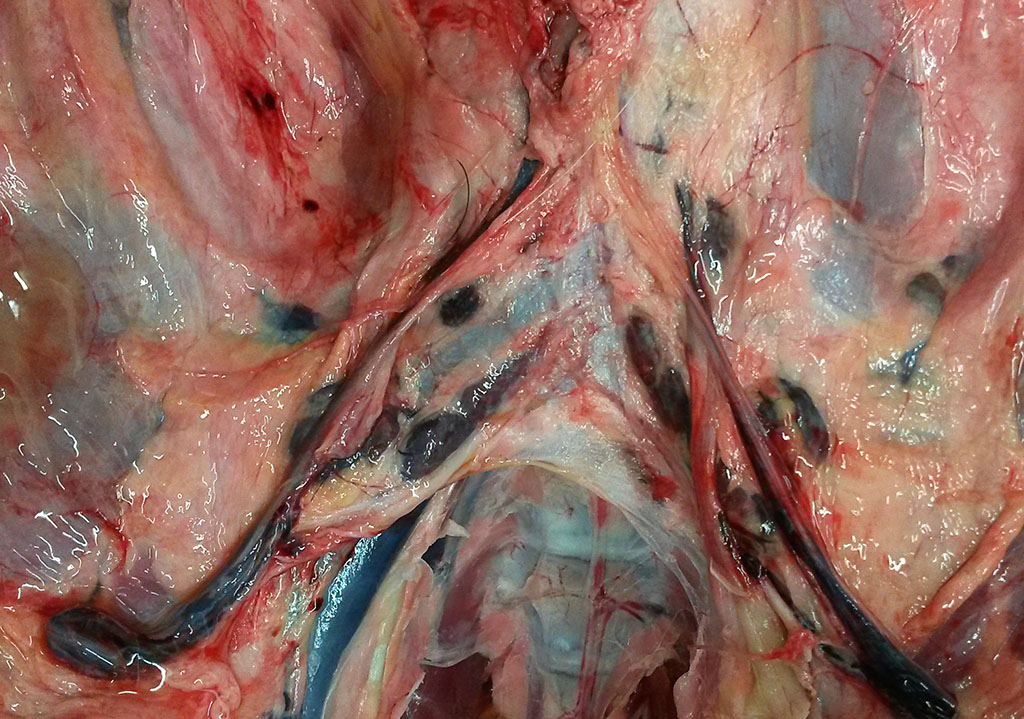
Gross Pathology:The pig was in a moderate body condition (26 kg body weight). Signs of autolysis were mild (due to the low temperature weather conditions). The pig was moderately infected with lice. The carcass was severely cyanotic (especially snout and ears) and all lymph nodes were severely hyperemic. Multiple abscesses were seen in the mandibular lymph nodes. The lung was not collapsed and heavy. The cranial lobes and multifocally the diaphragmatic lobes of the lung were consolidated and dark red with multiple small abscesses. The trachea was filled with large amounts of pus. The liver was severely hyperemic and had multiple irregularly distributed 1 to 5 mm in diameter foci of necrosis. The spleen was severely enlarged. Embolic suppurative nephritis was present in both kidneys. There were multiple foci of necrosis in the adrenals. The stomach was severely hyperemic. The gastrointestinal tract including the colon was almost empty. No signs of gun-shot wounds or injuries caused by an accident (e.g. collision with a car) were detected.
Laboratory results: Salmonella Choleraesuis was isolated from multiple tissues.
PCRs were negative for European swine fever virus, African swine fever virus, pseudorabies virus, rabies virus and PCV-2.
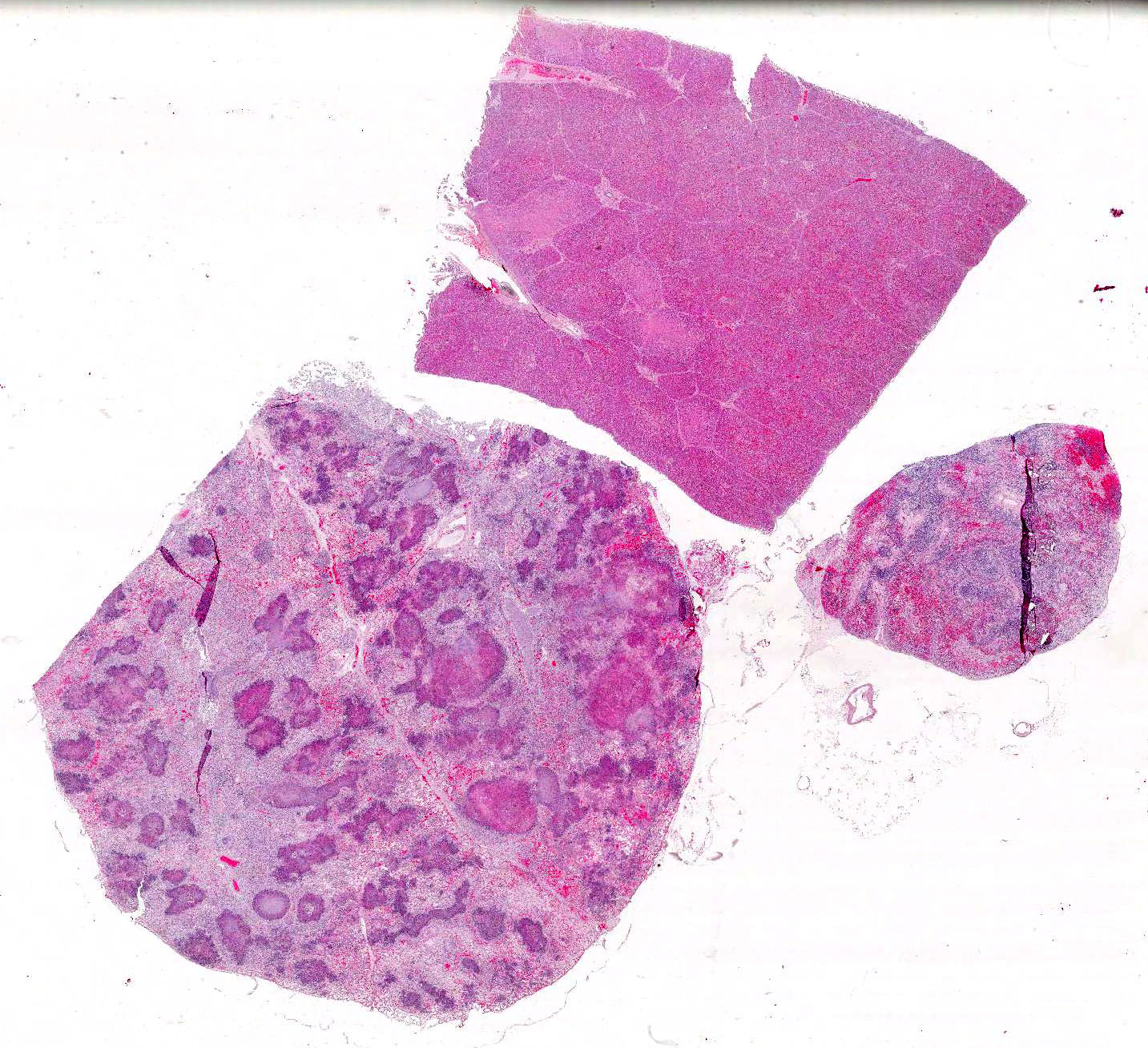
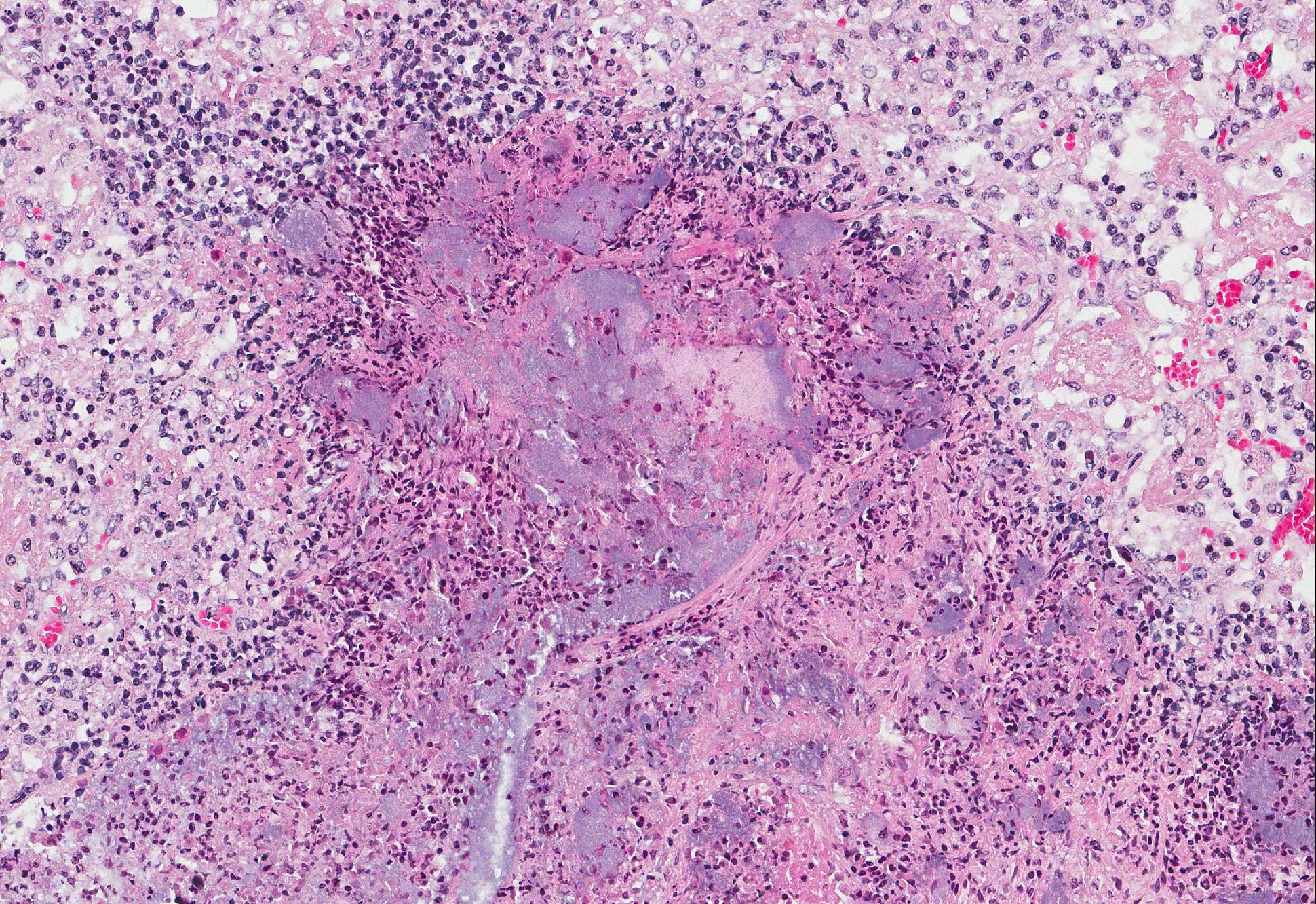
Microscopic Description: Lung: There are numerous foci of necrosis which appear to be associated with the airways. They are irregular shaped, of variable size and sometimes confluent. They are surrounded by predominantly neutrophilic infiltrates. Alveoli of the adjacent pulmonary tissue are filled with fibrin, contain few neutrophils and occasionally erythrocytes. The alveolar septae are severely hyperemic. Some contain fibrin thrombi. Numerous bacterial colonies are present throughout the pulmonary tissue (both gram positive and gram negative). Salmonellae are frequently detected in the periphery of the necrotic foci and in bacterial emboli in small blood vessels.
Lymph node (mediastinal): The subcapsular region is dilated by hyperemia, hemorrhages and infiltrates of neutrophils and histiocytes. There are multifocal areas of necrosis and neutrophilic infiltration most likely associated with larger blood vessels. Small blood vessels are obliterated by fibrin thrombi and bacterial emboli. Numerous bacterial emboli are present throughout the section. Lymphoid follicles are severely depleted
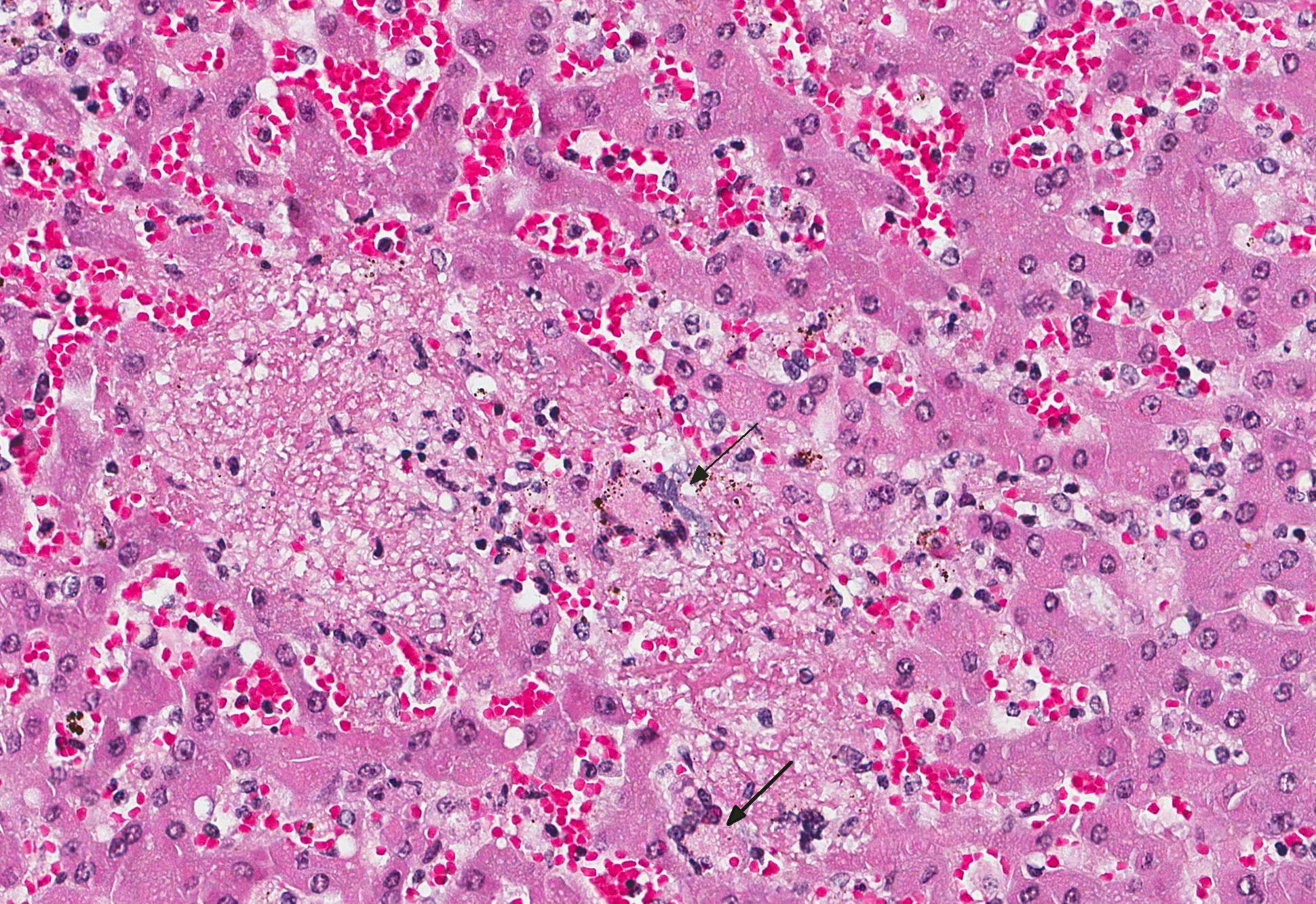
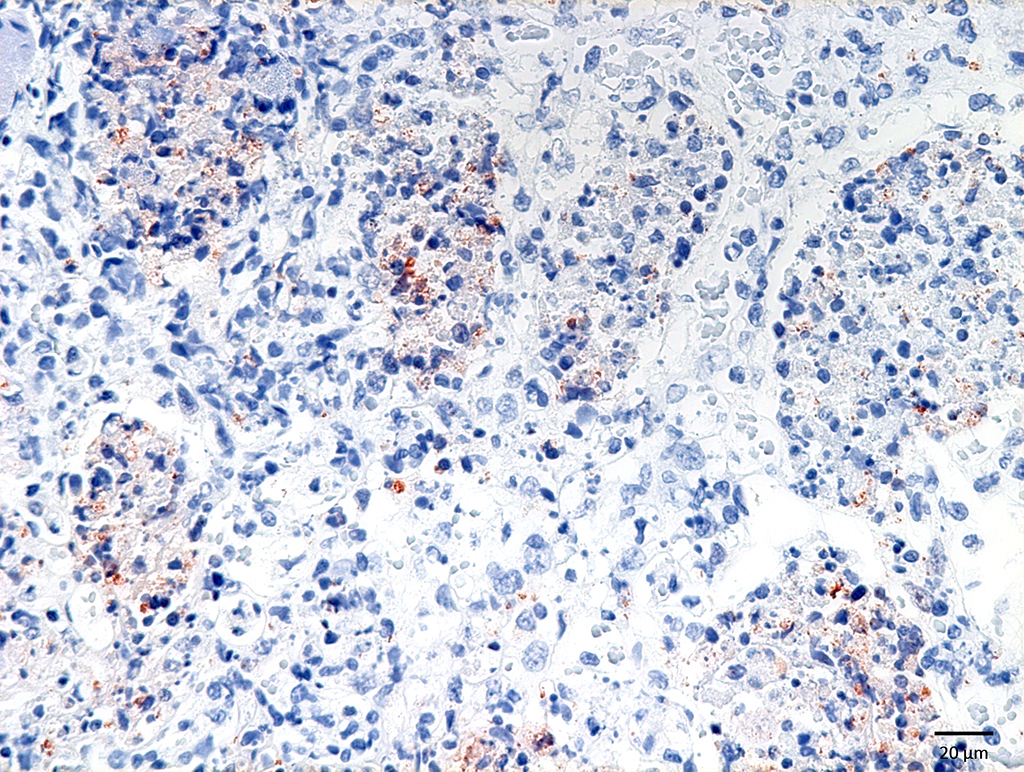
Liver: Many small and irregularly distributed foci of necrosis are present throughout the hepatic tissue. Some consist of pure coagulation necrosis; others have foci of hemorrhage or are infiltrated by few neutrophils or histiocytes. The liver is severely hyperemic. In addition to erythrocytes, leucocytes and fibrin are present in hepatic arteries and veins. Sinusoids are dilated by hyperemia and leukocytostasis. Bacterial colonies and emboli occur in a multifocal distribution. They stain positive for salmonella LPS by IHC. There are a few areas with loss of hepatic tissue structure and infiltration of large numbers of long bacilli without inflammatory reaction.
Contributors Morphologic Diagnosis:
Lung: Severe, diffuse acute fibrinopurulent and necrotizing bronchopneumonia with bacterial colonies, fibrin thrombi and bacterial emboli in small blood vessels.
Lymph node (mediastinal): Severe purulent and necrotizing lymphadenitis with severe hyperemia, hemorrhages and lymphatic depletion as well as fibrin thrombi and bacterial emboli in small blood vessels.
Liver: Severe multifocal acute necrotizing hepatitis with bacterial emboli in small blood vessels.
Contributors Comment: Groups of gram-negative bacteria were seen in all tissues examined. IHC using a primary antibody against salmonella LPS (MCA2832, Bio-Rad) identified them as salmonella. In the lung, gram-positive coccoid bacteria were found in addition. Gram-labile long slender bacilli in the liver were interpreted as post mortem invasion.
Salmonella are gram-negative, aerobic to facultative anaerobic, motile, facultative intracellular bacilli. They represent one genus in the family Enterobacteriaceae. The genus Salmonella encompasses more than 2500 species, subspecies and serovars. Salmonella (S.) enterica subspecies enterica has the largest number of serovars (>1500) including the most important animal and human pathogens, e.g. S. enterica subspecies enterica Choleraesuis, abbreviated as S. Choleraesuis.
Pathogenic salmonella can be differentiated in host adapted serovars (e.g. S. Choleraesuis in pigs, S. Dublin in cattle, S. Typhi in humans) and serovars with a wide host spectrum (e.g. S. Typhimurium and many others). Host-adapted salmonella cause severe generalized disease both in young and adult hosts; serovars with a wide host spectrum cause predominantly enterocolitis in young animals.
Three syndromes are associated with salmonella infections of swine11: (1) septicemic salmonellosis usually associated with S. Choleraesuis, (2) acute or chronic enterocolitis and rectal stricture associated with S. Typhimurium and (3) ulcerative enterocolitis and/or caseous tonsillitis and lymphandenitis associated with S. Typhisuis. In addition, clinically in apparent infections by numerous other salmonella serovars are possible.4
The main reservoir for S. Choleraesuis is the intestinal tracts of pigs.4 Persistent carriers occur and shedding can be activated by stress. During acute disease, up to 106 S. Choleraesuis/g feces are excreted. Salmonella remain infectious for 3 months in wet and for 6 months in desiccated feces. Therefore contact to other pigs or environment contaminated by pigs are the main sources of infection. S. Choleraesuis is rarely associated with contamination of carcasses or pork products. Thus, it is unusual as cause of human disease. If human disease occurs, it is severe.
The main route of infection is the alimentary tract including the tonsils. Irrespective of the route of inoculation, S. Choleraesuis was most frequently recovered from the ileocecal junction, ileocolic lymph node, cecal contents, tonsil, lung and colon.3 After crossing the mucosal barrier, infection of macrophages and dendritic cells occurs. Survival in phagocytes is an important feature of virulence and allows systemic bacterial dissemination.11
Lesions in septicemic salmonellosis are mainly associated with endothelial damage and microvascular thrombosis due to endotoxins, cytokines released by endotoxin activity and bacterial emboli.3,9,11 This results in congestion of many organs, widespread hemorrhages, often as petechiae, and pulmonary edema and causes fatal disease. At necropsy, thrombosis of capillaries and venules in the dermal papillae of the skin, glomerulonephritis, paratyphoid nodules (ranging from coagulative necrosis to granulomas) in the liver, splenomegaly with small lymphoid follicles, but histiocytic infiltrates and meningoencephalitis are common findings. The injury to the capillaries in pulmonary interalveolar septae may result in a fulminant fibrinous bronchopneumonia.
Differential diagnoses are other causes of generalized disease with high fever (classical swine fever, African swine fever) or septicemia (pasteurellosis, streptococcal infection, erysipelas) or fibrinous pneumonia (e.g. Actinobacillus pleuropneumoniae infection).
During the 1950s and 1960s, S. Choleraesuis was the predominant serovar isolated from pigs worldwide. At the present time, it is still highly prevalent in domestic pigs in North America and Asia, but rare in Australia and Western Europe.1,7 There are a few case reports of S. Choleraesuis in wild boars in Germany.6,8,10,12 A survey of perished wild boars submitted to the regional diagnostic laboratory of Thuringia, Germany, detected S. Choleraesuis in 20% (24 pigs) of the pigs examined.5 Further characterization of the isolates revealed that distinct isolates were circulating in herds of wild boars separated by natural barriers or arterial roads. There appears to be a higher susceptibility of young animals, because predominantly shoats and juvenile pigs were affected. The percentage of carriers and shedders without clinical signs remained unresolved. S. Choleraesuis infection of wild boar may serve as reservoir for domestic pigs especially if kept outdoors. Attention should be paid to game meet inspection to reduce health risks for hunters and persons preparing and consuming pork from wild boars.
JPC Diagnosis: 1. Lung: Bronchopneumonia, necrotizing multifocal to coalescing, severe with numerous large bacterial (do we want to specify / i.e.; coccobacilli?) colonies, Central European boar, porcine.
2. Liver: Hepatitis, necrotizing, multifocal, mild to moderate with occasional large bacterial colonies.
3. Lymph node: Lymphadenitis, necrotizing, acute, multifocal, marked with hemorrhage and occasional bacterial colonies.
Conference Comment: This case provided a comprehensive presentation of Salmonella choleraesuis in a wild boar. There were a few atypical microscopic findings noted during the conference. First, there were rare multinucleated giant cells in the liver and lymph node which is not a typical finding salmonellosis. Second, the pattern of pneumonia in the lung appears to be bronchocentric, which is unusual for S. Choleraesuis, which often presents as an embolic interstitial pneumonia.11 Affected animals may develop bronchopneumonia as a result of secondary bacterial opportunists but in this case IHC using a primary antibody against salmonella LPS (MCA2832, Bio-Rad) demonstrated the presence of bacilli in large numbers within multiple airways. An alternate expalantion was proposed by the moderator, who suggested the bronchogenic appearance may have resulted from an outgrowth of inflammation within the adjacent bronchial vascular tree. Finally, conference participants noted that it is unusual to see such large colonies of Salmonella sp. within lesions. However, after much discussion, attendees were resigned to the fact that under favorable circumstances excessively large burdens of any bacteria could form colonies in affected tissues.
The contributors comments abovesuperbly reviews the serovars of Salmonella sp. affecting domestic animals, porcine specific serovars and their clinical syndromes, pathogenesis, and gross findings, and the history and distribution of salmonellosis in wild boars in Europe. Salmonellae are often found in the intestinal lamina propria and regional lymph nodes of poultry and cattle, which are important reservoirs for the organism. . Salmonella choleraesuis is a common co-infection with hog cholera (classical swine fever), and was originally thought to be the causative agent of classical swine fever, with which it shares many gross lesions.11
During the discussion on the pathogenesis of salmonellosis, the conference moderator pointed out that the cause of all of the microscopic changes associated with salmonellosis is due to endothelial damage resulting from endotoxin release. Endotoxin (lipopolysaccharide or LPS) is a virulence factor encoded on Salmonella pathogenicity islands (SPI-2), which are clusters of plasmid genes that can be shared among bacterial colonies. In addition to LPS, SPIs also code for other virulence factors such as fimbriae, motility, and other secreted proteins. Endotoxins induce the release ofcytokines which leads to thrombosis of capillaries and venules (Shwartzman reaction), hemorrhage, and eventual ischemic necrosis of visceral organs. Additionally, endotoxins help bacteria evade phagocytosis, decreases lysis within the phagolysosome, and renders complement useless, thus perpetuating survival and replication of the organism.2
Friedrich-Loeffler-Institut
Federal Research Institut for Animal Health
Suedufer 10, D-17493
Greifswald-Insel Riems, Germany
References:
1. Eddicks M, Hausleitner R, Eddicks L, et al. Detection of Salmonella choleraesuis var. Kunzendorf in a fattening pig with septicaemic salmonellosis. A case report. Tierarztl Praxis. 2016; 44:381-387.
2. Gelberg HB. Alimentary system and the peritoneum, omentum, mesentery, and peritoneal cavity. In: Zachary JF, ed. Pathologic Basis for Veterinary Disease. 6th ed. St. Louis, MO: Elsevier; 2016: 377-378.
3. Gray JT, Fedorka-Cray PJ, Stabel TJ, Ackermann MR. Influence of inoculation route on the carrier state of Salmonella choleraesuis in swine. Vet Microbiol. 1995; 47(1-2):43-59.
4. Griffith RW, Schwartz KJ, Meyerholz DK. Salmonella. In: Eds. Straw BE, Zimmerman JJ, D´Allaire S, Taylor DJ. Diseases of Swine. 9th ed. Asia: Blackwell Publishing; 2006:739-754.
5. Methner U, Heller M, Bocklisch H. Salmonella enterica subspecies enterica serovar choleraesuis in a wild boar population in Germany. Eur J Wildl Res. 2010; 56:493-502.
6. Müller M, Weber A, Tucher R, Naumann L. Case report: Salmonella choleraesuis as a cause of hematogenous osteomyelitis in a wild boar (Sus scrofa). Tierärztl Umschau. 2004; 59:700-702.
7. Pedersen K, Sørensen G, Löfström C, Leekitcharoenphon P, Nielsen B, Wingstrand A, Aarestrup FM, Hendriksen RS, Baggesen DL. Reappearance of Salmonella serovar choleraesuis var. Kunzendorf in Danish pig herds. Vet Microbiol. 2015; 176(3-4):282-291.
8. Plötner J, Bussemer R, Otta J, Schmidt O, Winkler H. Zu einer salmonella-cholerae-suis-Infektion im Schwarzwildbestand zweier benachbarter Jagdgebiete. Mh Vet Med. 1979; 34:860-861.
9. Reed WM, Olander HJ, Thacker HL. Studies on the pathogenesis of Salmonella typhimurium and Salmonella choleraesuis var kunzendorf infection in weanling pigs. Am J Vet Res. 1986; 47(1):75-83.
10. Schulze C, Neumann G, Grütze I, Engelhardt A, Mirle C, Ehlert F, Hlinak A. Case report: Porcine circovirus type 2 infection in an European wild boar (Sus scrofa) in the state of Brandenburg, Germany. Dtsch Tierarztl Wochenschr. 2003; 110(10):426-428.
11. Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In Maxie MG, ed. Jubb, Kennedy, and Palmer`s Pathology of Domestic Animals. Vol 2, 6th ed. St. Louis, MO: Elsevier; 2016:170-173.
12. Weber A, Broos H, Wachowitz R, Heil G, Schultze-Rhonhof J. Salmonella choleraesuis in wild boar (Sus scrofa) in Northern Bavaria. Tierärztl Umschau. 1990; 45: 411-414.