Signalment:
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Generally, adenoviruses are host specific and are transmitted by the fecal-oral route or by direct contact via oro-nasal secretions. Adenoviral infection in reptiles is reported with and without concurrent disease and in the majority of communications only individual animals are afflicted. The most common changes observed in association with an adenoviral infection include nonspecific clinical signs like wasting and anorexia, accompanied by necrosis and inflammatory changes in the gastrointestinal tract and the liver.(5,7-9,11,12) Others observed nephritis (7) or neurological signs like head tilt and circling,(8) but there are also cases of sudden death without prior signs of illness described.(9)
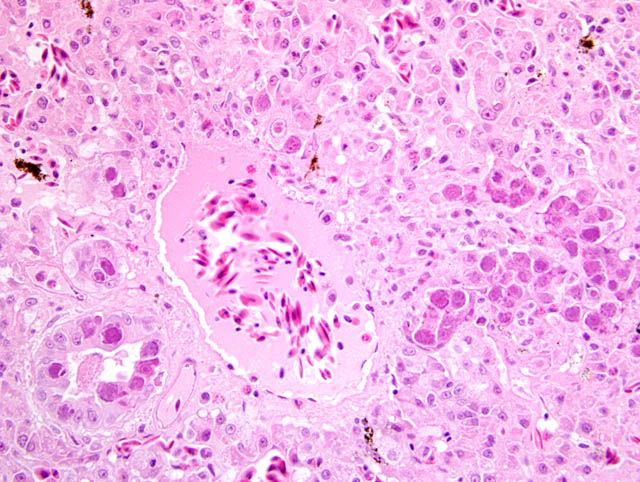
Additionally, the two animals investigated showed intranuclear inclusions in the tubular epithelial cells of the kidney identical to those seen in the liver associated with a moderate, multifocal, lympho-histiocytic interstitial nephritis. Furthermore, a moderate, multifocal, lympho-histiocytic, interstitial pancreatitis with numerous intranuclear inclusion bodies in epithelial cells of the pancreatic duct was detected. The microscopically visible intranuclear inclusion bodies are formed during the replication phase inside the nuclei of affected host cells. Using routine H&E stain, they are typically basophilic to amphophilic.(5,8,9,11,12) The virions form paracrystalline arrays, often causing severe condensation and margination of the host cell chromatin. Finally, they are released by cell lysis.(6) In order to substantiate the diagnosis, the products of the PCR were sequenced and compared to published sequences of reptilian adenoviruses in the GenBank by use of BLAST.(10)
JPC Diagnosis:
Conference Comment:
The contributor provides a useful synopsis of the pathogenesis of adenoviral infections in animals. In general, adenoviruses interact with host cells in one of three characteristic ways:(2)
- Lytic infection: involves complete viral replication and host cell lysis, as described by the contributor
- Latent or chronic infection: lymphoid tissue is often the site of latency
- Oncogenic transformation (e.g., production of sarcomas in hamsters by simian and human adenoviruses): viral DNA is integrated into host cell DNA and T antigens are produced, but no infectious virions are produced
Recently, a fifth genus has been added to the Adenoviridae family: the Ichtadenovirus genus, containing Sturgeon adenovirus A as its sole species.(4) An example of canine adenovirus is available in WSC 2008-2009, Conference 3, case I, and an example of falcon adenovirus is available in WSC 2007-2008, Conference 23, case III.
References:
2. Cheville NF, Lehmkuhl H: Cytopathology of viral diseases. In: Ultrastructural Pathology: The Comparative Cellular Basis of Disease, ed. Cheville NF, 2nd ed., pp. 338-343, Wiley-Blackwell, Ames, IA, 2009
3. Davison AJ, Wright KM, Harrach B: DNA sequence of frog adenovirus. J Gen Virol 81:2431-2439, 2000
4. International Committee on Taxonomy of Viruses (Internet): Virus taxonomy. Available from: http://www.ictvonline.org, 2008
5. Jacobson ER, Kopit W, Kennedy FA, Funk RS: Coinfection of a Bearded Dragon, Pogona vitticeps, with adenovirus- and dependovirus-like viruses. Vet Pathol 33:343-346, 1996
6. Jacobson ER: Viruses and viral diseases of reptiles. In: Infectious Diseases and Pathology of Reptiles, ed. Jacobsen ER, pp. 401-402, CRC Press, Boca Raton, FL, 2007
7. Julian AF, Durham PJ: Adenoviral hepatitis in a female bearded dragon (Amphibolorus barbatus). N Z Vet J 30:59-60, 1982
8. Kim DY, Mitchell MA, Bauer RW, Poston R, Cho DY: An outbreak of adenoviral infection in inland bearded dragons (Pogona vitticeps) coinfected with dependovirus and coccidial protozoa (Isospora sp.). J Vet Diagn Invest 14:332-334, 2002
9. K+�-+bber-Heiss A, Benetka V, Filip T, Benyr G, Schilcher F, Pallan C, M+�-�stl K: Erstmaliger Nachweis einer Adenovirus-Infektion bei einer Bartagame (Pogona vitticeps AHL, 1926) in +�-�sterreich. Wiener Tier+�-�rztliche Monatsschrift, 93:68-72, 2006
10. Moormann S, Seehusen F, Reckling D, Kilwinski J, Puff C, Elhensheri M, Wohlsein P, Peters M: Systemic Adenovirus Infection in Bearded Dragons (Pogona vitticeps): Histological, Ultrastructural and Molecular Findings. J Comp Pathol, 141:78-83, 2009
11. Perkins LE, Campagnoli RP, Harmon BG, Gregory CR, Steffens WL, Latimer K, Clubb S, Crane M: Detection and confirmation of reptilian adenovirus infection by in situ hybridization. J Vet Diagn Invest 13:356-368, 2001
12. Ramis A, Fern+�-�ndez-Bellon H, Maj³ N, Mart+�-�nez-Silvestre A, Latimer K, Campagnoli R: Adenovirus hepatitis in a boa constrictor (Boa constrictor). J Vet Diagn Invest 12:573-576, 2000
13. Wellehan JF, Johnson AJ, Harrach B, Benk+�-� M, Pessier AP, Johnson CM, Garner MM, Childress A, Jacobson ER: Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the atadenoviruses. J Virol 78:13366-13369, 2004