CASE I: A21-106 (JPC 4167686)
Signalment:
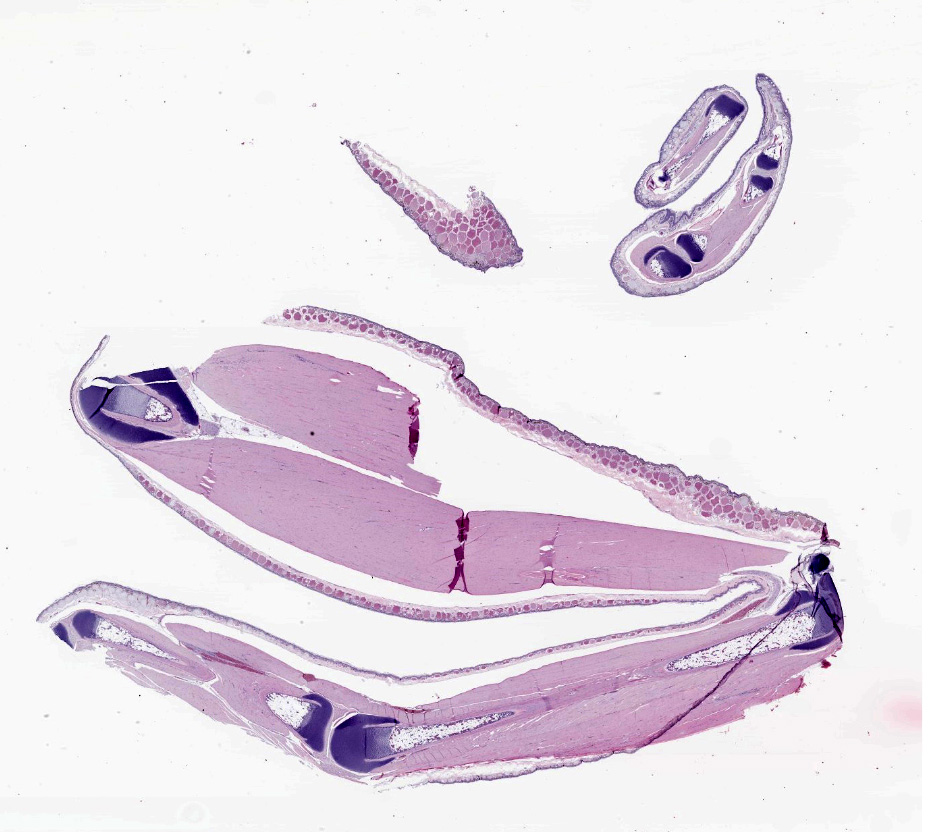
9-month-old White's tree frogs (Ranoidea caerulea, formerly Litoria caerulea).
Multiple individuals (three females and one male) submitted.
History:
Sudden deaths of multiple cohabitants over the course of a few weeks.
Gross Pathology:
No relevant gross findings.
Laboratory Results:
None provided.
Microscopic Description:
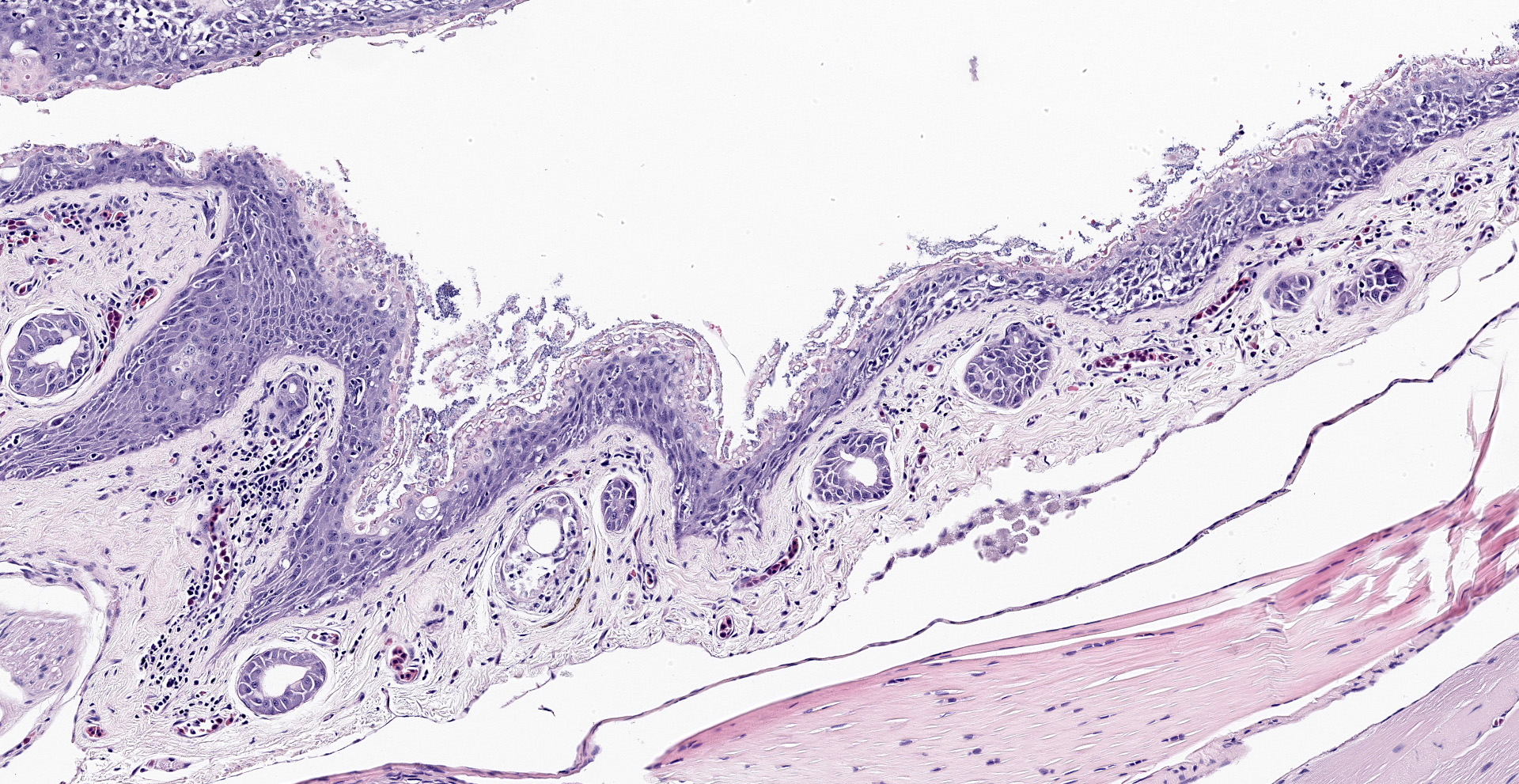
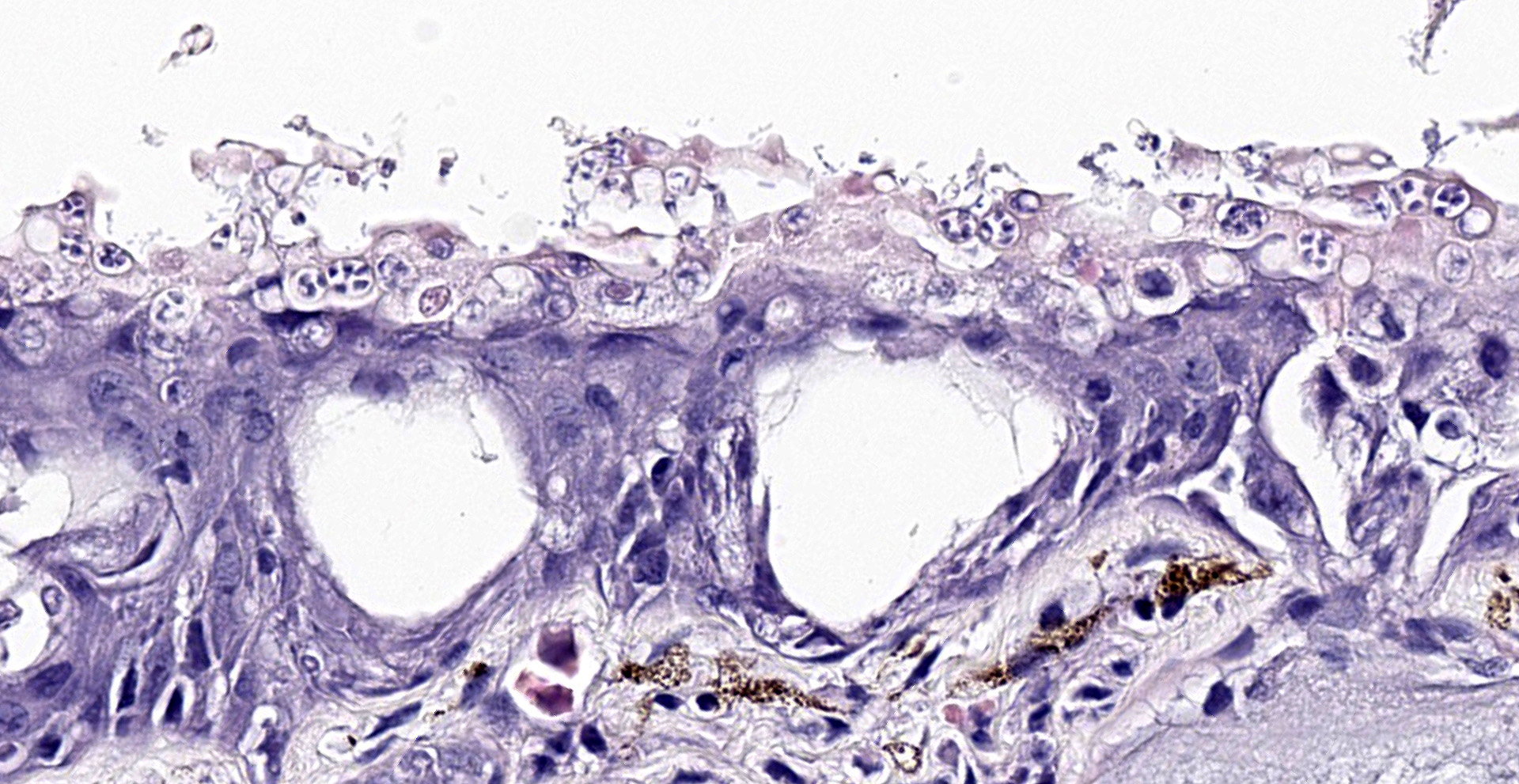
The epidermis is diffusely hyperplastic with multifocal to coalescing areas of orthokeratotic hyperkeratosis. The hyperkeratotic stratum corneum is diffusely expanded by 1-5 layers of retained keratinocytes. Retained keratinocytes contain moderate to high numbers of intracellular, round, fungal structures measuring 5-10um in diameter (thalli). Thalli variably contain roughly round, basophilic zoospores measuring 2-3um in diameter. Infrequently, spike-like projections extend from thalli toward the skin surface (discharge tubules). Minimal lymphocyte exocytosis is observed in the affected epidermis. Few lymphocytes, histiocytes, and granulocytes are scattered in the superficial dermis.
Contributor's Morphologic Diagnoses:
Skin: Epidermal hyperplasia and hyperkeratosis, diffuse, moderate to marked, chronic, active, with high numbers of intracorneal thalli, morphology consistent with Batrachochytrium dendrobatidis.
Contributor's Comment:
Batrachochytrium spp., the causative agents behind the infectious disease chytridiomycosis, are fungal pathogens responsible for mass morbidity and mortality in amphibian populations worldwide.11 Two species have thus far been implicated in global amphibian population declines; Batrachochytrium dendrobatidis (Bd), which has been identified in over 50 countries and over 500 amphibian species,1 and Batrachochytrium salamandrivorans (Bsal), which primarily affects salamanders and newts.8 Bd infects the keratinized layer of the epidermis. The disease is therefore primarily seen in post-metamorphic adults, but Bd can infect the keratinized oral discs of tadpoles.7
Amphibian skin is actively involved in maintenance of homeostasis, gas exchange, and electrolyte balance. Through infection of the superficial epidermis, Bd impairs the function of amphibians' epidermal ion transport channels, disrupting osmoregulation and resulting in systemic electrolyte imbalance.15 Mechanisms by which Bd causes systemic electrolyte imbalance include direct disruption of epidermal intercellular junctions via production of virulence-associated proteins;3 influencing the expression of genes involved in collagen, fibrinogen, elastin, keratin, and ion channel production;15 and possibly direct inhibition of sodium transport channels.4, 17 Disease in affected animals typically does not elicit a strong immune response, and it has been hypothesized that Bd may also impair host immune responses.6 Overall, disease course and outcome are influenced by a variety of pathogen, host, and environmental factors, but the resulting hyponatremia, hypokalemia, and hypochloremia eventually lead to arrhythmia, cardiac arrest, and death.4, 11, 15
Affected amphibians typically first present with nonspecific signs of disease such as anorexia and lethargy. Some affected animals exhibit abnormal or excessive shedding patterns.10 Sudden death without any premonitory signs is also possible.11
Gross lesions can include rough skin texture, greyish discoloration of the skin, erythema, ulceration, abnormal shedding, and thinning and sloughing of the skin.4, 11 However, in cases of sudden death, no gross lesions may be appreciated. Histologically, fungal thalli are identified in the stratum corneum and stratum granulosum with associated hyperkeratosis and epidermal hyperplasia. Lesions are typically most pronounced on the feet and ventral body.2 Thalli are round and 5-15um in diameter with a variably present projection extending toward the skin surface known as a discharge tubule. Depending on their stage of development, thalli may be empty and clear-staining with only thin internal septa visible or filled with few, round, basophilic zoospores that are 2-3um in diameter.11 Inflammatory cell infiltrates are not commonly observed in association with thalli.11
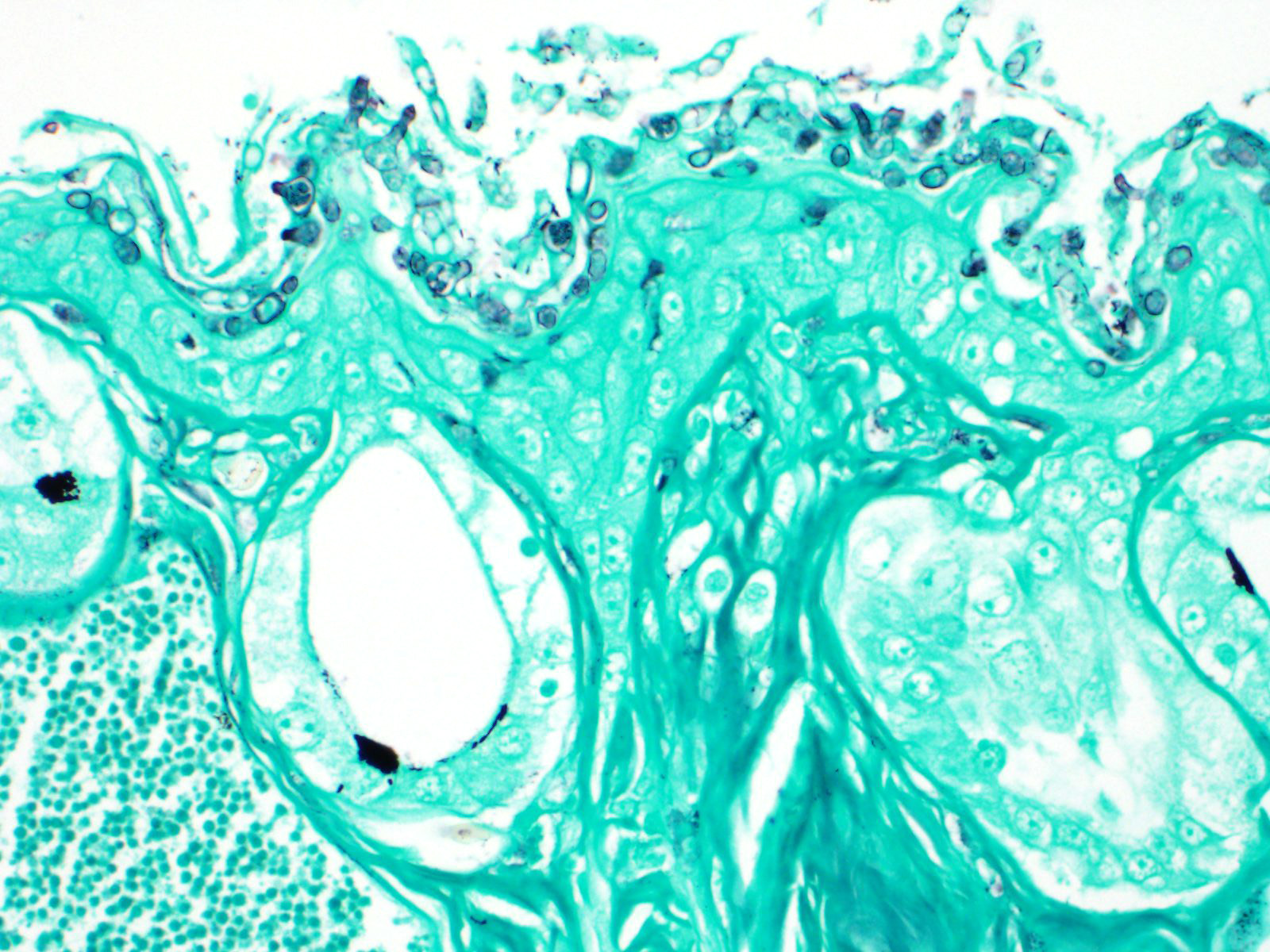
Histochemical stains such as Gomori's methenamine silver stain (GMS) and Periodic-acid Schiff for fungus stain (PAS-F) can be used to highlight intracorneal thalli. Wet mounts and skin scrapings may also aid in diagnosis of Bd.
Contributing Institution:
University of Florida
College of Veterinary Medicine
Department of Comparative, Diagnostic, and Population Medicine
JPC
Diagnosis:
Skin, epidermis: Hyperplasia and hyper-keratosis, multifocal to coalescing, moderate to severe, with numerous fungal thalli and zoospores, etiology consistent with Batrachochytrium dendrobatidis.
JPC Comment:
All fungi, including those of division Chytridiomycota (commonly known as 'chytrids'), share a common aquatic ancestor from which metazoans and fungi both originated approximately 1.2 billion years ago. Given their early divergence from other fungi approximately 750 million years ago, chytrids retain many features from their common ancestor and are suited for survival in aquatic environments, such as the production of mobile ciliated zoospores. Chytrids also feature several features shared by all fungi, such as a hyphal-like structure known as a ?thallus?, which is associated with a feeding structure known as a rhizoid that absorbs nutrients while also anchoring the fungus to the substrate.9
Chytrids are found in both aquatic and terrestrial environments. Many, such as Batrachochytrium dendrobatidis, parasitize animals while others target algae and plants or are saprophytic. These organisms play a major role in aquatic food chains by parasitizing large inedible algae while also producing zoospores that are a food source for zooplankten. Anaerobic chytrids are also components of the rumen microbiome. With the help of specialized organelles known as hydrosomes, these organisms facilitate the breakdown of resilient carbohydrates in plant tissues, which in turn aids volatile fatty acid and microbial proteins production by additional constituents of the rumen microbiome.9
Most chytrid species exhibit a similar life cycle beginning with a zoospore that subsequently progresses to a thallus and then a sporangium. Zoospores range from 2-10 µm in diameter and typically have a single posterior cilium used for propulsion in aquatic environments. Zoospores are non-mitotic have limited energy stores derived from lipids and carbohydrates allocated during zooporogenesis, which are often visible microscopically as lipid droplets. Upon finding itself in a suitable environment, the motile zoospore encysts by retracting its cilia and forms a fungal cell wall. Previously inhibited by cilia, the now free centrioles are utilized for cell division. As the cyst germinates, a primitive germ tube forms, which subsequently arborizes into a hyphal-like rhizoidal system. Depending on the species, a sporangioum forms at the site of the nucleus, which is either located within the cyst or from a separate location within a rhizoid as the result of nuclear migration. Within the developing sporangium, the nucleus undergoes multiple divisions without cytoplasmic division, forming a coenocyte. This is followed by ciliogenesis, by which centrioles are converted into basal bodies and the cilia are formed. Nuclei are then individually encapsulated by membrane invaginations. Following the completion of zoosporogenesis, mature zoospores are released via one or more pores (discharge papillae) within the cell wall.9
Batrachochytrium dendrobatidis (Bd) and Batrachochytrium salamandrivorans (Bsal) are the etiologic agents of chytridiomycosis, an emerging infectious amphibian disease responsible for mass die-offs worldwide. As of a 2019 report14, chytridiomycosis has contributed to the decline of 501 amphibian species (6.5% of described amphibian species), of which 90 are extinct in the wild and 124 have experienced a >90% decline. Of the 501 species affected, all but one species decline were attributed to Bd. These figures constitute the greatest known loss of biodiversity attributed to a pathogen. In comparison, other well-known pathogens such as white-nose syndrome in bats (Pseudogymoascus destructans) and West Nile virus are responsible for the declines of six species of bats and 23 species of birds, respectively. Anurans, which comprise 89% of amphibian species, are most commonly affected and represent 93% of the species in severe decline, with 45% of severe declines and extinctions occurring in the neotropical genera Atelopus, Craugastor, and Telmatobius.14
A study15 evaluating a chronically Bd-infected population compared to a Bd-free population of the endangered Australian frog Litoria verreauxii alpina found a higher proportion of males and females in infected populations reached sexual maturity at a younger age than non-infected populations. In males, this resulted in reduced size at maturity and overall may demonstrate an adaptive evolutionary shift in response to selection pressure induced by high Bd-induced mortality.15
In contrast to Bd which is predominantly associated with anuran hyperkeratosis, Bsal is a pathogen of salamanders and newts and is characterized by multifocal superficial erosions and deep ulcerations in the skin. Although anurans are seemingly unaffected, they can act as carriers and likely facilitate the spread of the disease as the result of inter/intraspecies contact associated with anthropogenic trade. This entity recently spread from Asia into Europe, decimating the fire salamander (Salamandra salamandra) population in The Netherlands in 2013.5 Hosting one of the most diverse salamander populations in the world, The United States also imported 750,000 salamanders from 2010-2014, creating a high risk of Bsal introduction.12 As a result, the US Fish and Wildlife Commission banned the import of 201 species of salamanders into the United States in 2016. Although Bd is reported worldwide, Bsal has not yet been reported in North America.5
Conference participants noted the dermis and underlying musculature are separated by abundant clear space. A possible explaination for this clear space is lymph sac edema, however, the moderator suggested the lack of associated flocculent material is more consistent with processing artifact.
References:
1. Berger L, Roberts AA, Voyles J, Longcore JE, Murray KA, Skerratt LF. History and recent progress on chytridiomycosis in amphibians. Fungal Ecol. 2016 Feb 1;19:89?99.
2. Berger L, Speare R, Daszak P, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci U S A. 1998 Jul 21;95:9031?9036.
3. Brutyn M, D'Herde K, Dhaenens M, et al. Batrachochytrium dendrobatidis zoospore secretions rapidly disturb intercellular junctions in frog skin. Fungal Genetics and Biology. 2012 Oct 1;49:830?837.
4. Campbell CR, Voyles J, Cook DI, Dinudom A. Frog skin epithelium: Electrolyte transport and chytridiomycosis. Int J Biochem Cell Biol. 2012 Mar 1;44:431?434.
5. Farrer RA. Batrachochytrium salamandrivorans. Trends Microbiol. 2019;27(10):892-893.
6. Fites JS, Ramsey JP, Holden WM, et al. The Invasive Chytrid Fungus of Amphibians Paralyzes Lymphocyte Responses. Science. 2013 Oct 18;342:366?369.
7. Marantelli G, Berger L, Speare R, Keegan L. Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pac Conserv Biol. 2004;10:173?179.
8. Martel A, Blooi M, Adriaensen C, et al. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science. 2014 Oct 31;346:630?631.
9. Medina EM, Buchler NE. Chytrid fungi. Curr Biol. 2020;30(10):R516-R520.
10. Nichols DK, Lamirande EW, Pessier AP, Longcore JE. Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. J Wildl Dis. 2001 Jan;37:1?11.
11. Pessier AP. Amphibia. In: Terio KA, McAloose D, St Leger J, eds. Pathology of Wildlife and Zoo Animals. Academic Press; 2018:1136.
12. Richgels KL, Russell RE, Adams MJ, White CL, Grant EH. Spatial variation in risk and consequence of Batrachochytrium salamandrivorans introduction in the USA. R Soc Open Sci. 2016;3(2):150616.
13. Rosenblum EB, Poorten TJ, Settles M, Murdoch GK. Only skin deep: shared genetic response to the deadly chytrid fungus in susceptible frog species. Mol Ecol. 2012;21:3110?3120.
14. Scheele BC, Pasmans F, Skerratt LF, et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science. 2019;363(6434):1459-1463.
15. Scheele BC, Skerratt LF, Hunter DA, et al. Disease-associated change in an amphibian life-history trait. Oecologia. 2017;184(4):825-833.
16. Voyles J, Young S, Berger L, et al. Pathogenesis of Chytridiomycosis, a Cause of Catastrophic Amphibian Declines. Science. 2009 Oct 23;326:582?585.
17. Wu NC, Cramp RL, Ohmer MEB, Franklin CE. Epidermal epidemic: unravelling the pathogenesis of chytridiomycosis. J Exp Biol. 2019 Jan 27;jeb.191817.