Signalment:
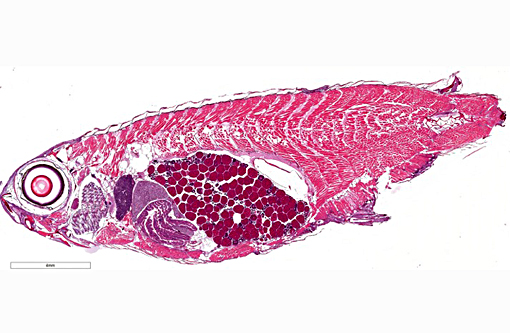
Gross Description:
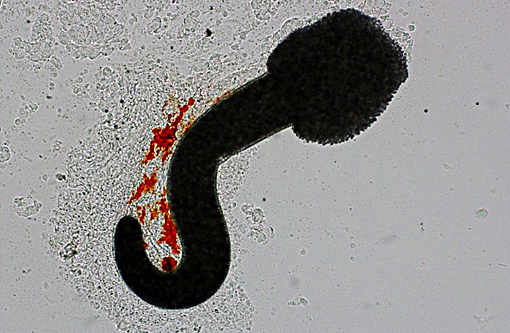
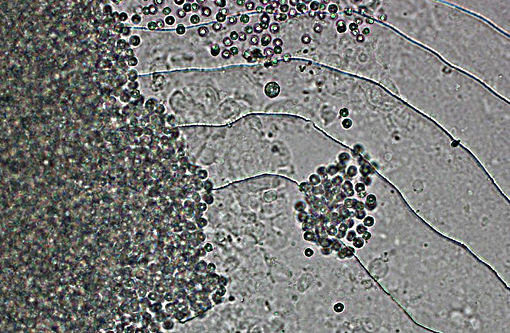
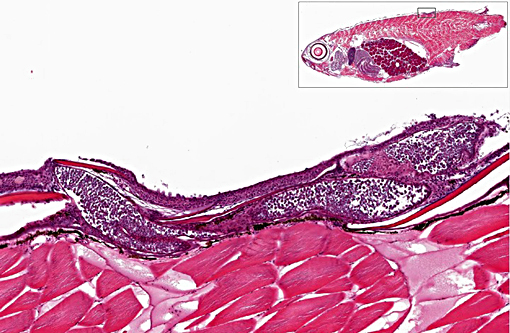
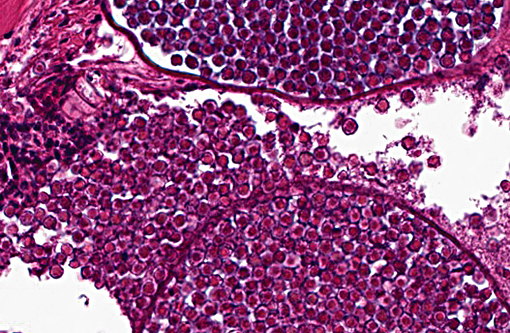
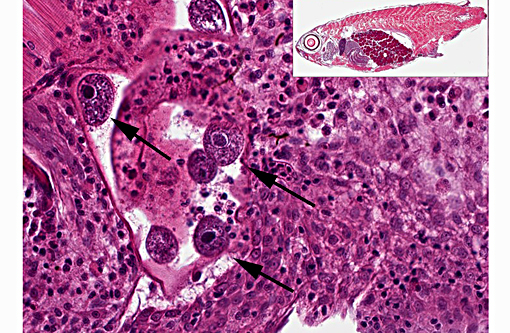
Histopathologic Description:
Morphologic Diagnosis:
1. Skin, dorsum, ventrum, base of anal and caudal fins: Dermatitis, granulocytic, histiocytic, multifocal, subacute, mild to moderate with multiple intralesional mesomycetozoal cysts and luminal spores (Dermocystidium spp.)
2. Skin, base of anal fin: Dermatitis, granulocytic, histiocytic, focal, subacute, moderate with intralesional protozoa (Tetrahymena spp.)
Lab Results:
Condition:
Contributor Comment:
Phylogenetic analysis of the 18s small subunit rDNA genes indicates this class contains 10 different genera of parasitic and saprophytic microbes including: Amoebidium, Anurofeca, Dermocystidium, Ichthyophonus, Pseudoperkinsus, Psoros-permium, Rhinosporidium, Sphaerosoma and two currently unnamed agents clone LKM51 and rosette agent.(2)
The majority of mesomycetozoea are pathogens of aquatic species, specifically fishes and invertebrates with Rhinosporidium a noteable exception. The life cycles of these organisms have not been completely documented. In vitro, both Dermocystidium and the rosette agent develop uniflagellated zoospores which could serve as a method of transmission and infection.(2) Waterborne transmission of Dermocystidium has been documented.(4)
There are currently 14 recognized species of Dermocystidium which all cause pathogenic infection in fishes and aquatic invertebrates.(7) The skin and gills are the primary sites of infection, though visceral lesions have also been reported.(5) The most diagnostic feature on cytology and histology is the presence of cysts (sporocysts) containing the characteristic spherical spore (endospore) stage with a large central vacuole (refractile body).(3) There are four previous reports of Dermocystidium infection in Paracheirodon genus fishes (cardinal tetra, Paracheirodon axelrodi and neon tetra, P. innesi) with two providing histopathology similar to what is presented in this case.(1,6) In the two most recent reports the agent was confirmed by 18S rDNA PCR to be Dermocystidium salmonis, a pathogen previously reported in Pacific salmon species. Both reported lesions to be predominantly epidermal and most severe along the anterior body and fins.(1,6) The histologic lesions and distribution of the infection in the fish presented here are similar to these two previous reports, which suggests Dermocystidium salmonis infection in this group of cardinal tetra. One cardinal tetra from this group was submitted for 18S rDNA PCR and diagnostic confirmation; however, results were not available at the time of case submission.
In recent case reports as well as in this case infection occurred in mixed species tanks, but only Paracheirodon genus fishes were affected. This may indicate a sensitivity of this genus to Dermocystidium infection. A source for infection was not clear in the current case. There were no recent changes in water source and there was no prior history of this infection in other tanks. There had been recent (6 weeks prior) addition of other genera of fishes to the impacted tank following 30 day quarantine, however there had not been a recent introduction of Paracheirodon spp. It is not clear from the current literature if a carrier state in other fishes is possible. The focal Tetrahymena infection in this case was believed to be opportunistic and incidental.
JPC Diagnosis:
Conference Comment:
Some conference participants identified the ciliate as a trichodinid due to its similar size compared with a tetrahymenid, but as the moderator pointed out, Trichodina spp. dont penetrate the skin whereas tetrahyminids do. Trichodinids, while also ciliates, generally cause a relatively mild disease in healthy fish but can result in significant losses in young fish, particularly when secondary bacterial infections are present or in cases of debilitation due to other causes. Tetrahymenids are often opportunistic invaders, as seen in this case, but can also invade internal organs and result in lethal infections in severe cases. Infection has been referred to as guppy disease due to its preference for infecting guppies. Tetrahymena sp. can also cause disease in catfish, common carp, and rainbow trout secondary to skin damage and invasion of internal organs.(3)
References:
1. Langenmayer MC, Lewisch E, Gotesman M, et al. Cutaneous infection with Dermocystidium salmonis in cardinal tetra, Paracheirodon axelrodi (Schultz, 1956). J Fish Dis. 2014; 38(5):503-506.
2. Mendoza L, Taylor JW, and Ajello L. The Class Mesomycetozoea: Heterogeneous group of microorganisms at the animal-fungal boundary. Ann Rev Microbiol. 2002; 56:315-355.
3. Noga EJ. Fish Disease: Diagnosis and treatment. 2nd ed. Ames, IA: Wiley-Blackwell; 2010: 137-141, 174-175.
4. Olson RE, Dungan CF, and Hold RA Water-borne transmission of Dermocystidium salmonis in the laboratory. Dis Aquat Org. 1991; 12:41-49.
5. Roberts RJ. The mycology of teleosts. In: Roberts RJ, ed. Fish Pathology. Chicheste, UKr: Wiley-Blackwell; 2012: 383-401.
6. Westmoreland LSH, Hadfield CA, Clayton LA, et al. Mesomycetozoea in cardinal tetras (Paracheirodon axelrodi) and green neon tetras (Paracheirodon simulans). In: Proceedings of the IAAAM, 46th Annual Conference. Chicago: April 6-10, 2015.
7. Index Fungorum; www.indexfungorum.org. Accessed January 13, 2016.