Signalment:
Gross Description:
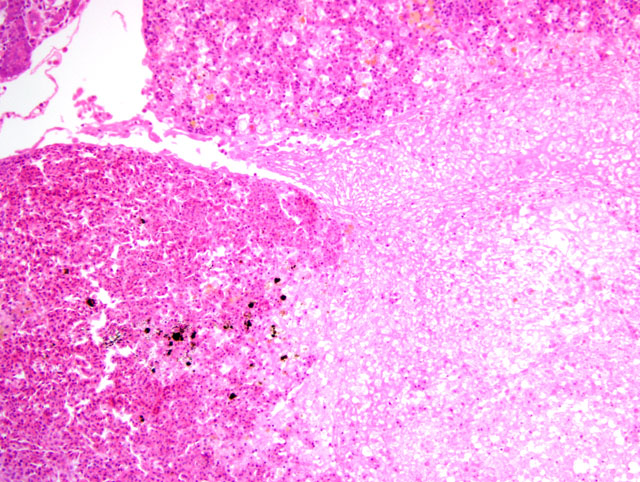
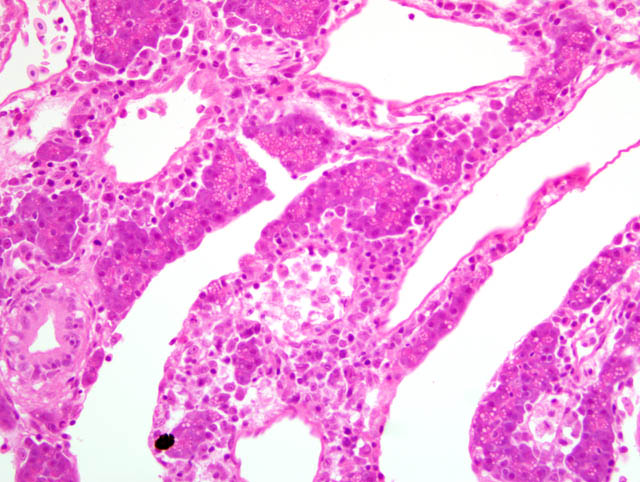
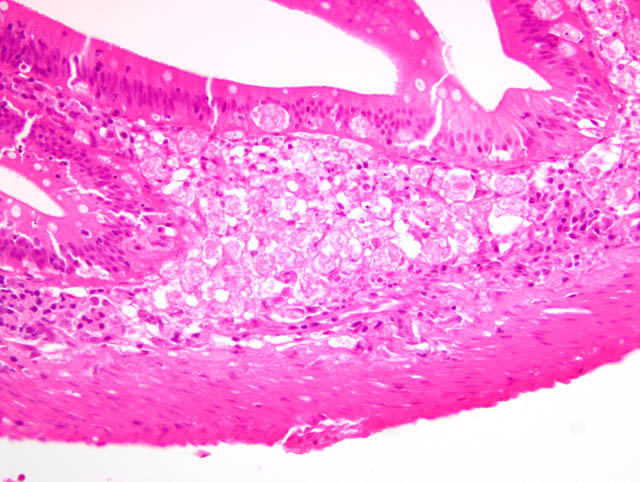
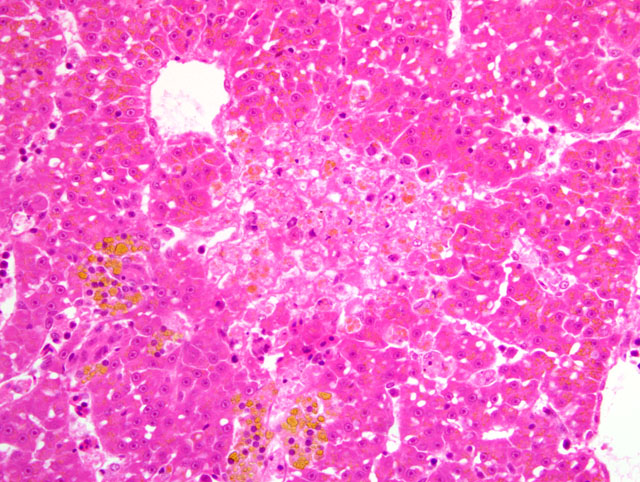
Histopathologic Description:
Morphologic Diagnosis:
1. Renal hematophoietic tissue: Necrosis, multifocal, locally extensive, moderate to severe, with eosinophilic intranuclear inclusion bodies, etiology consistent with herpesvirus hematopoietic necrosis, Goldfish, ornamental, Carassius auratus.
2. Spleen: Necrosis, multifocal, moderate, with eosinophilic intranuclear inclusion bodies.Â
3. Pancreas: Necrosis, multifocal, mild to moderate, with eosinophilic intranuclear inclusion bodies.
4. Intestine: Necrosis, submucosa and mucosa, segmental, multifocal, mild to severe, with eosinophilic intranuclear inclusion bodies in epithelial cells, also inflammation, lymphoplasmacytic, segmental, moderate.Â
Lab Results:
Condition:
Contributor Comment:
A herpesvirus, designated goldfish hematopoietic necrosis virus (GFHNV), has been isolated from affected goldfish and shown to reproduce HVHN experimentally.(9) Herpesvirus-like particles can be detected by electron microscopy in affected tissues.(1,5,9,10) HVHN of goldfish has pathological similarities to carp nephritis and gill necrosis (CNGN), which affects koi carp (Cyprinus carpio) and is caused by koi herpesvirus (carp nephritis and gill necrosis virus).(6) GFHNV does not affect koi carp (1) and koi herpesvirus does not infect goldfish(6). Outbreaks of CNGN were first observed in cultured koi carp in Israel and North America in 1998 (6) and have subsequently been reported in Japan, Indonesia and Europe, including the United Kingdom.(3,7) HVHN and CNGN are both characterized by multifocal necrosis of renal hematopoietic, splenic, pancreatic and intestinal tissue, with variable histological lesions in the gills, oropharynx and skin.(1,5,6,9,10) Necrosis, lamellar fusion and epithelial hyperplasia are observed in the gills of koi carp with CNGN, similar to gill lesions in goldfish with HVHN.(6) Koi herpesvirus can be detected in tissues from affected koi carp by electron microscopy and polymerase chain reaction.(2,4,6) Comparisons of the genomes of koi herpesvirus isolates from outbreaks of CNGN in different regions of the world by restriction fragment length polymorphism, protein gel electrophoresis and DNA hybridization have demonstrated that most isolates are nearly identical and are likely to have been derived from a common source.(2-4)
In outdoor ponds in Japan and North America, GFHNV usually causes disease in cultured goldfish in spring and autumn (fall), when the temperature of the water is in the range of 15 to 25 degrees Celsius.(5,9) Outbreaks of CNGN caused by koi herpesvirus have been reported on farms with water temperatures of 17 to 25 degrees Celsius.(6,7) Experimentally infected koi carp are susceptible to disease in water with a temperature range from 18 to 28 degrees Celsius.(3) Goldfish affected by major outbreaks of HVHN have usually been less than one year of age, with some epidemics occurring in juveniles less than two months of age, although goldfish of all ages may be affected.(1,5,9,10) Two-month-old goldfish affected by HVHN in North America were 25 to 40 mm long and weighed two to three grams.(5) A single goldfish diagnosed with HVHN in Australia weighed approximately five grams.(10) Koi carp of all ages may be affected by CNGN.(5) In the case of HVHN reported here, a single adult goldfish 90 mm long and weighing seven grams was affected. It could be speculated that this goldfish developed HVHN after first exposure to GFHNV following introduction of an infected fish. Alternatively, the goldfish may have harboured latent GFHNV that was reactivated following the stress caused by the introduction of new fish to the aquarium tank. Outbreaks of mortality due to HVHN in 20 to 25-day-old goldfish fry, as well as associated mortality in adult brood goldfish, were associated with the introduction of new fish to a hatchery in Taiwan.(1)
JPC Diagnosis:
1. Pancreas: Pancreatitis, necrotizing, multifocal, moderate to marked, with intranuclear inclusion bodies and acute serosistis.
2. Intestine, lamina propria and submucosa: Necrosis, multifocal, with intranuclear inclusion bodies.
3. Spleen; and renal hematopoietic tissue: Necrosis, multifocal, with intranuclear inclusion bodies.
4. Liver: Hepatocellular degeneration and necrosis, multifocal, mild to moderate, with few intranuclear inclusion bodies.
Conference Comment:
- Cyprinid herpesvirus-1: carp pox herpesvirus
- Cyprinid herpesvirus-2: hematopoietic necrosis herpesvirus of goldfish
- Cyprinid herpesvirus-3: koi herpesvirus
Despite substantial variation among the slides with respect to the organs and lesions represented, participants readily recognized a systemic herpesviral infection primarily targeting hematopoietic and lymphoid tissues. During the conference, the conference moderator cautioned participants that pancreatic necrosis in fish is often very difficult to distinguish from autolysis. However, the contributor in this case provided histologic sections from particularly well-preserved tissues as a good example of this important entity, and further complimented the slides with a thorough review. Additionally, conference participants commented on finding serositis in many slides, which is not surprising given the widespread distribution of pancreatic tissue in fish. Furthermore, most slides contain abundant intra- and extracellular accumulations of yellow to orange, granular to globular material throughout the liver. Conference participants discussed various potential pigments, including hemosiderin, hematoidin, bile, melanin, and lipofuscin. In this case, an abundance of iron-containing pigment (i.e. hemosiderin) was demonstrated by the presence of intense blue staining with the Prussian blue reaction. Staining using the Halls bile method was negative.
References:
2. Gilad O, Yun S, Andree KB, Adkison MA, Zlotkin A, Bercovier H, Eldar A, Hedrick RP: Initial characteristics of koi herpesvirus and development of a polymerase chain reaction assay to detect the virus in koi, Cyprinus carpio koi. Dis Aquat Organ 48:101-108, 2002
3. Gilad O, Yun S, Adkison MA, Way K, Willits NH, Bercovier H, Hedrick RP: Molecular comparison of isolates of an emerging fish pathogen, koi herpesvirus, and the effect of water temperature on mortality of experimentally infected koi. J Gen Virol 84:2661-2667, 2003
4. Gray WL, Mullis L, LaPatra SE, Groff JM, Goodwin A: Detection of koi herpesvirus DNA in tissues of infected fish. J Fish Dis 25:171-178, 2008
5. Groff JM, LaPatra SE, Munn RJ, Zinkl JG: A viral epizootic in cultured populations of juvenile goldfish due to a putative herpesvirus etiology. J Vet Diagn Invest 10:375-378, 1998
6. Hedrick RP, Gilad O, Yun S, Spangenberg JV, Marty GD, Nordhausen RW, Kebus MJ, Bercovier H, Eldar A: A herpesvirus associated with mass mortality of juvenile and audult koi, a strain of common carp. J Aquat Anim Health 12:44-57, 2000
7. Hoole D, Bucke D, Burgess P, Wellby I: Diseases of Carp and Other Cyprinid Fishes, pp. 48-49. Fishing News Books, Blackwell Science, Oxford, UK, 2001
8. International Committee on Taxonomy of Viruses (Internet): Virus taxonomy. Available from: http://www.ictvonline.org, 2008
9. Jung SJ, Miyazaki T: Herpesviral haematopoietic necrosis of goldfish, Carassius auratus (L.) J Fish Dis 18:211-220, 1995
10. Stephens FJ, Raidal SR, Jones B: Haematopoietic necrosis in a goldfish (Carassius auratus) associated with an agent morphologically similar to herpesvirus. Aust Vet J 82:167-169, 2004