Signalment:
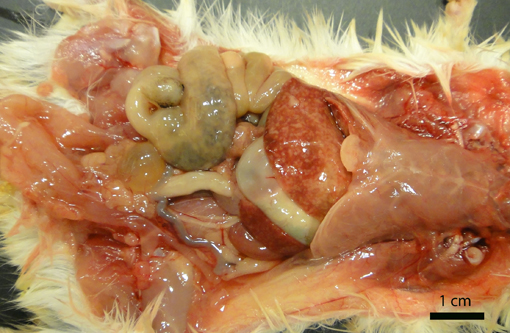
Gross Description:
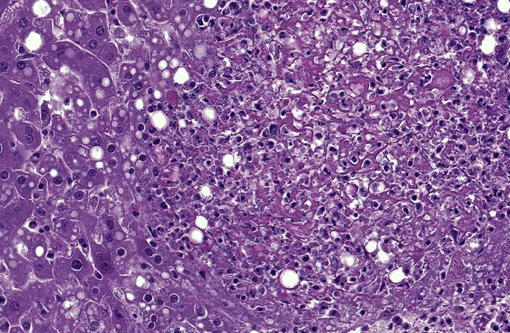
Histopathologic Description:
Other significant histologic findings (not present in the slide provided) include:
Spleen: Splenitis, necrotizing, acute, multifocal severe with intralesional short bacilli
Lymph nodes, mesenteric and mandibular: Lymphadenitis, necrotizing, acute, diffuse, severe
Intestine: Enterocolitis, necrotizing, acute, multifocal to transmural, severe
Bone marrow: Myelitis, necrotizing, acute, multifocal, moderate
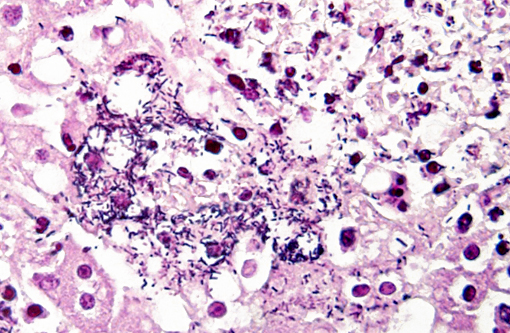
Gram stain: Bacteria are Gram positive.Â
Morphologic Diagnosis:
Lab Results:
Aerobic culture, liver: Many Listeria spp.Â
Anaerobic culture, liver: No anaerobes isolated.
Lung or liver from 3 other jirds were sent for aerobic culture and revealed many Listeria spp. in two, and many Listeria monocytogenes in one.Â
Condition:
Contributor Comment:
LM has more than 11 serotypes and almost all domestic animal infections are caused by serotypes 1/2a, 1/2b and 4b.(8) It is an intracellular pathogen of macrophages, neutrophils and epithelial cells.(8) Mobile genetic elements in Listeria spp. include pathogenicity islands (LIPI-1 and LIPI-2) and plasmids.(6) LIPI-1 encodes for a cluster of virulence factors including hemolysins (such as listeriolysin O)(11,13) and actin-polymerase factors, necessary for the intracellular survival and spread of the organism.(6) LIPI-2 is specific for Listeria ivanovii and thought to be responsible for restriction of its host specificity to ruminants and for its lack of typical tropism for the central nervous system observed in LM.(6) Other important virulence factors of LM include the internalins, surface proteins which internalize with E-cadherin to overcome the intestinal, placental and blood-brain barriers.(8)
The three recognized syndromes in domestic animals, that seldom overlap, are infection of the pregnant uterus with abortion, septicemia with miliary visceral abscesses, and encephalitis.(8) In rodents and small mammals the septicemic form predominates.(9) In our jirds, out of the 6 cases in which histology was performed, the organs most commonly affected were liver (6/6), spleen (6/6), large intestine (5/6), small intestine (4/6), lymph nodes (3/4), bone marrow (2/6), lung (1/6) and brain (1/6). Septicemic listeriosis is characterized by multisystemic bacterial colonization with multifocal areas of necrosis or microabscess formation.(8) In our case, the lesions were very acute and only small numbers of neutrophils were recognized within the lesions and adjacent blood vessels. Jirds are gerbil-like rodents(12) that belong to the family Gerbillinae. Gerbils are used as models for Listeria monocytogenes infection and naturally succumb to the disease.(4)
LM is a zoonotic pathogen that can be transmitted through alimentary sources (food-borne) and direct contact.(11) Food-borne transmission of the bacterium was suspected in our jirds, but food was not available for testing. This disease has been previously reported in bushy tailed jirds and the source of infection was similarly not elucidated in that mortality event.(12) Diagnosis was reached by histopathology and microbiology. Gram stains confirmed the presence of gram-positive bacteria in the lesions. Other diagnostic methods that could be of use include immunohistochemistry and PCR.Â
JPC Diagnosis:
Conference Comment:
The pathogenicity island LIPI-1 encodes the bacterial surface protein surface protein actA which is vital for Listerias ability to colonize. Once the bacterial population reaches a sufficient size within a single cell, it propels into adjacent cells via actin polymerization of this protein and forms membrane protrusions known as pseudopods. The pseudopods penetrate into adjacent cells forming double-membrane endocytotic phagocytic vesicles which are subsequently lysed by the virulence factor listerolysin-O among others.(13) Transfer of the pseudopod into adjacent cells is facilitated by expression of phosphatidylserine, the well-known surface phospholipid important in both the coagulation cascade and efferocytosis. Effectively, Listeria spp. exploits efferocytosis to promote its dissemination. Therefore, a likely target of effective antimicrobial therapy may be phosphatidylserine or its binding receptor for this and other similar bacterial pathogens.(3)
References:
1. Akanbi OB, Breithaupt A, Polster U, et al. Systemic listeriosis in caged canaries (Serinus canarius). Avian Pathol. 2008;37(3):329-332.
2. Crespo R, Garner MM, Hopkins SG, Shah DH. Outbreak of Listeria monocytogenes in an urban poultry flock. BMC Veterinary Research 2013;9:204.
3. Czuczman MA, Fattouh R, Van Rijn JM, et al. Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread. Nature. 2014;509(7499):230-234.
4. Disson O, Nikitas G, Grayo S, Dussurget O, Cossart P, Lecuit M. Modeling human listeriosis in natural and genetically engineered animals. Nature Protocols. 2009;4(6):799-810.
5. Girling SJ, Fraser MA. Listeria monocytogenes septicaemia in an inland bearded dragon (Pogona vitticeps). Journal of Herpetological Medicine and Surgery. 2004;14:6-9.
6. Gyles C, Boerlin P. Horizontally transferred genetic elements and their role in pathogenesis of bacterial disease. Vet Pathol.2014;51:328-340.
7. Langohr IM, Ramos-Vara JA, Wu CC, Froderman SF. Listeric meningoencephalomyelitis in a cougar (Felis concolor): Characterization by histopathologic, immunohistochemical and molecular methods. Vet Pathol. 2006;43(3):381-383.
8. Maxie GM, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Saunders Elsevier; 2007:405408.
9. Morner T. Listeriosis. In: Williams ES, Baker IK, eds. Infectious Diseases of Wild Mammals. 3rd ed. Ames, IA: Iowa State University Press. 2001:502.
10. Nichols M, Takacs N, Ragsdale J, Levenson D, Marques C, Roache K, et al. Listeria monocytogenes infection in a sugar glider (Petaurus breviceps)- New Mexico, 2011. Zoonoses Public Health. 2014: doi: 10.1111/zph.12134.
11. Oliver HF, Wiedmann M, Boor KJ. Environmental reservoir and transmission into the mammarlian host. In: Goldfine H, Shen H, eds. Listeria Monocytogenes: Pathogenesis and Host Response. 1st ed. New York, NY: Springer; 2007:111.
12. Tappe JP, Chandler FW, Westrom WK, Liu S, Dolensek EP. Listeriosis in seven bushy-tailed jirds. J Am Vet Med Assoc. 1984;185(11):1367-1370.
13. Zachary JF. Mechanisms of microbial infections. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:192-195.