CASE IV: M20-05667 (JPC 4155412)
Signalment:
12 month old, mixed sex, diploid Sydney Rock Oysters (Saccostrea glomerata)
History:
A mortality event of approximately 42% occurred on an oyster farm in Autumn, with the worst affected oysters being the oldest, and those positioned on floating baskets compared with the lesser affected intertidal baskets. Water quality at the time was described as the ?worst in over 50 years?, persisting since heavy rains caused flood events after the area was impacted by large bush fires in Summer 2019-2020. Dead oysters presented as empty shells, and farmers noted remaining oysters were weaping a clear liquid.
Gross Pathology:
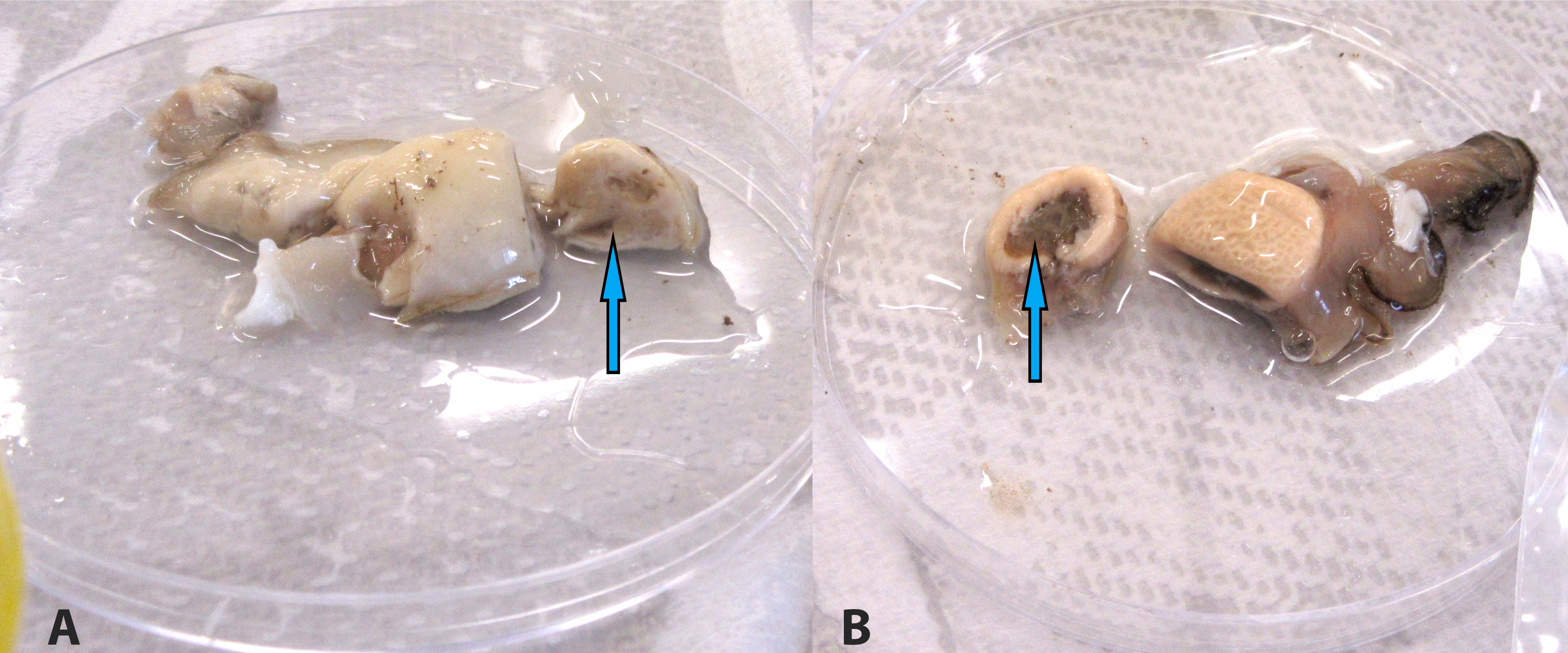
12 oysters were opened and examined. Most of the oysters appeared watery, with pale digestive glands. Non-affected oysters presented with normal dark brown digestive gland.
Laboratory results:
The digestive gland of each oyster was examined with impression smear, stained with Diff Quick. A sample of digestive gland was also collected and a QX (Marteilia sydneyi) PCR was performed. A strong correlation between smear results and DNA PCR results is consistently observed in our laboratory.
Microscopic Description:
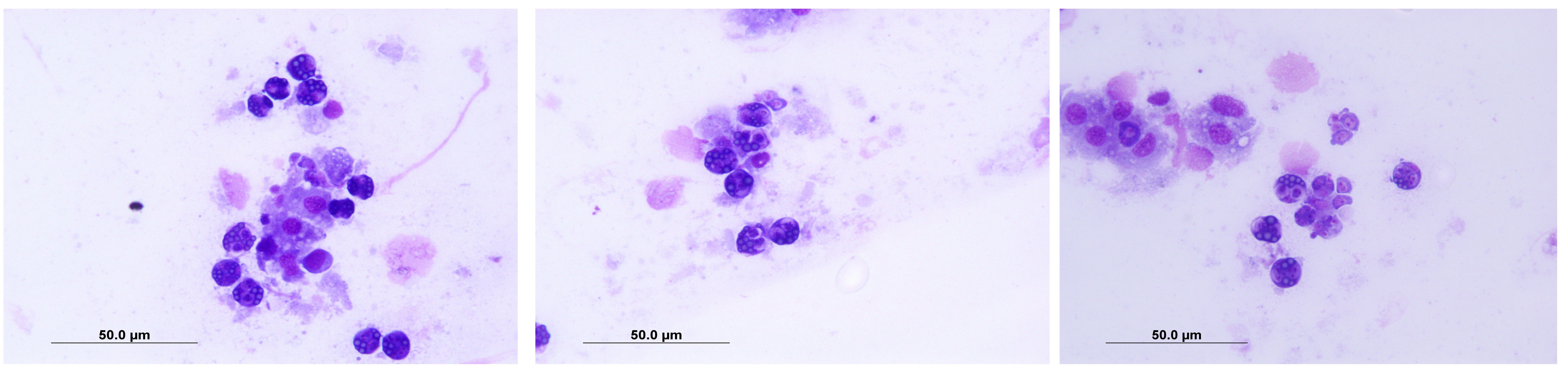
Impression smears, digestive gland, Sydney rock oysters.
There is moderate background granularity and amorphous debris. The smears contain nuclear material in streaks or clusters, and abundant sperm. There are numerous digestive gland epithelial cells with mild variation in size between 15-25µm, with large pale vacuolated cytoplasm and a loosely stained single nuclei. Occasionally there are basophilic secretory cells present, with dark blue cytoplasm and smaller size (approximately 5-10 µm diameter), and rare hemocytes. There are numerous various protozoal life cycle stages, including daughter cells (small 5-10 µm oval cells with blue cytoplasm), daughter cells containing secondary cells (larger 10-15 µm containing one or more plasmodia), immature sporonts and mature sporonts (containing numerous blue refractile bodies and two spores), admixed with cellular debris and ruptured nurse cells (sporangiosori).
Contributor?s Morphologic Diagnoses:
Digestive
gland smear: Marked parasitism with ruptured nurse cells, consistent with Marteilia
sydneyi infection, Sydney rock oyster (Saccostrea glomerata).
Contributor?s Comment:
The Australian bushfires in the summer of 2019-2020 burnt over 4.9 million hectares of land in New South Wales. Almost immediately following this event, there were heavy rainfalls, resulting in abundant ash runoff into rivers and estuaries. This ash particulate matter, combined with the fluctuations in salinity associated with flooding, may have contributed to many reports of mortalities in farmed aquaculture species, including the Sydney rock oysters, during this time. This mortality event was located in an estuary previously known to have QX, however active disease in Sydney rock oysters had not been diagnosed in the area for 13 years.
QX disease is caused by a protozoan parasite, Marteilia sydneyi (Phylum Paramyxea).5 The organism has been shown to have another host and potential environmental reservoir, the polychaete worm Nephtys australiensis.1 Since there is an environmental reservoir for the organism, it may be years between disease outbreaks in estuaries where the parasite is endemic.
Death from QX disease generally occurs in late summer to early spring (February-September)6 and may be associated with heavy rainfall3 and low salinity.4 Disease is diagnosed when the organism reaches sporulation in the digestive gland of Sydney rock oysters, thereby creating massive digestive gland lysis, necrosis and subsequent starvation and death of the oyster.6 Infected farms usually discover empty shell (dead oysters), with diseased oysters having pale, swollen digestive glands on gross examination. Individuals may also have depleted glycogen storage and gonad, with an overall watery appearance.6
Since M. sydneyi is present and endemic in a number of waterways in NSW, the diagnosis of disease requires the detection of sporulating forms, therefore digestive gland smear and histology is required to form a diagnosis. The detection of DNA alone by PCR is not a confirmatory test for the presence of disease. Digestive gland smears, stained with Diff Quick, are a relatively cost effective, fast and reliable diagnosis method, when compared with PCR or histopathology. In a study involving eighty oysters, results indicated cytology of digestive gland impression smears (96.88%) had a greater sensitivity of detecting QX disease than histology (86.11%).2
Contributing Institution:
https://www.dpi.nsw.gov.au/about-us/services/laboratory-services
JPC Diagnosis:
Impression smear, digestive gland: Epithelial cell degeneration with numerous intracellular sporonts consistent with Marteilia sp.
JPC Comment:
Based almost entirely upon only five species, global oyster production typically occurs in monocultures that are inherently vulnerable to disease epizootics. As a result, disease has significantly impacted oyster population dynamics since the development of oyster farming. Other than OsHV1 in Pacific oysters, the majority of oyster diseases are caused by protozoan parasites, including both QX disease and Winter Mortality Syndrome in Sydney rock oysters (Saccostrea glomerata).6
Sydney rock oyster farming is the fourth largest oyster aquaculture industry in the world and is New South Wales? largest aquaculture industry. However, production has declined over 40% since the 1970s, primarily due to the two aforementioned diseases. This trend has continued, with 3843 tons of edible oysters harvested in 2010-2011, 22% less ($4.7 million) than the previous year.6
QX (for Queensland Unknown) disease, also known as marteilosis, is the more serious of the two diseases with mortality rates reported to be ≥95% in some outbreaks.1,6 Caused by by Marteilia sydneyi, the initial outbreaks were first identified in the late 1970?s and described by Perkins and Wolf in 1976.6
The earliest infectious stage of M. sydneyi that can be identified in rock oysters is a uninucleate stem cell in the palps and gill epithelia, which suggests a ?free floating? parasitic stage likely enters the gills during filter feeding and then replicates in the gill epithelium. Once sufficient numbers of stem cells are generated, invade the subjacent connective tissue and disseminate throughout the oyster with the majority found in the digestive gland within the digestive tubule epithelium. Within the digestive gland, it forms a 2-celled plasmodium, which then undergoes further division, forming between 8 and 16 sporonts. An additional division occurs, with each sporonts dividing to form two spores, each with three concentric cells. These spores pass into the environment via the alimentary canal.6
The lifecycle of M. sydneyi once shed from infected oysters is unclear. Compared to the 3-10 month infection cycle within the host, spores are relatively short-lived once shed (7-35 days), which favors the theory of an intermediate host in the lifecycle of M. sydneyi.6 As noted by the contributor, previously unidentified and different morphologic stages of M. sydneyi have recently been identified within Nephtys australiensis, one of many polychaete nematodes found within the sediment near oyster leases. Although additional study is needed, it is possible the rock oyster and N. australiensis are the only two hosts required for the life cycle of this parasite.1
Winter Mortality Syndrome, the second major protozoan disease of Sydney rock oysters is caused by Bonamia roughleyi. As its name suggests, this disease predominantly occurs during the cooler months from June to August and is restricted to the cooler southern range of rock oysters. Mortality rates of up to 80% are common in affected areas and oysters are most susceptible in their third winter, just prior to reaching market size.6
References:
1. Adlard RD, Nolan MJ. Elucidating the life cycle of Marteilia sydneyi, the aetiological agent of QX disease in the Sydney rock oyster (Saccostrea glomerata). Intern. J. Parasitol. 2015 March 45; 419-426.
2. Adlard RD, Wesche SJ. Aquatic Animal Health Subprogram: Development of a disease zoning policy for Marteilia sydneyi to support sustainable production, health certification and trade in Sydney rock oyster. Fisheries Research and Development Corporation. Queensland Museum. 2005 2001/214.
3. Anderson TJ, Wesche S, Lester RJG. Are outbreaks of Marteilia sydneyi in Sydney rock oysters, Saccostrea commercialis, triggered by a drop in environmental pH? Aust. J. Marine Freshwater Res. 1994 45;1285-7.
4. Butt D, Shaddick K, Raftos D. The effect of low salinity on phenoloxidase activity in the Sydney rock oyster, Saccostrea glomerata. Aquaculture. 2006 251; 159-166.
5. Perkins FO, Wolf PH. Fine structure of Marteilia sydneyi sp. n. ? Haplosporidian pathogen of Australian oysters. J. Parasitol. 1976 August 62;4:528-538.
6. Raftos DA, Kuchel R, Aladaileh S, Butt D. Infectious microbial diseases and host defense responses in Sydney rock oysters. Front Microbiol. 2014;5:135. Published 2014 Apr 23.
7. Wolf PH. Life cycle and ecology of Marteilia sydneyi in the Australian oyster, Crassostrea commercialis. Marine Fisheries Review. 1979 41(1-2) 70-2.