CASE III: P963-20 (JPC 4168140)
Signalment:
4 ½ - 5 months old, female, Holstein veal calf (Bos taurus)
History:
Several milk-fed calves, in a herd of 800, developed multiple fractures at around 140 days of age. These calves were suddenly unable to get up or stand following a benign fall or after struggling to escape physical restraint for routine procedure. These calves had no prior locomotor problem and were in excellent body condition. They were fed a commercial feed supplemented in minerals and vitamins.
Gross Pathology:
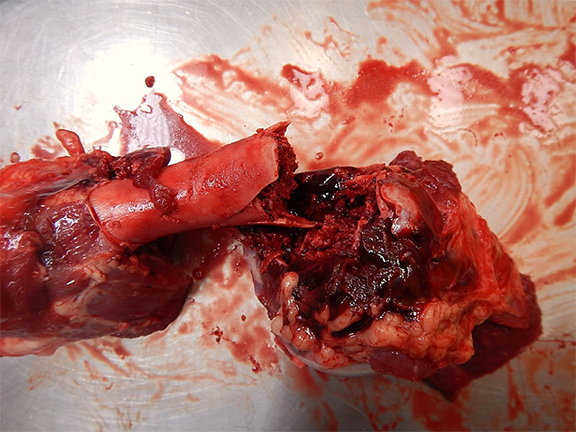
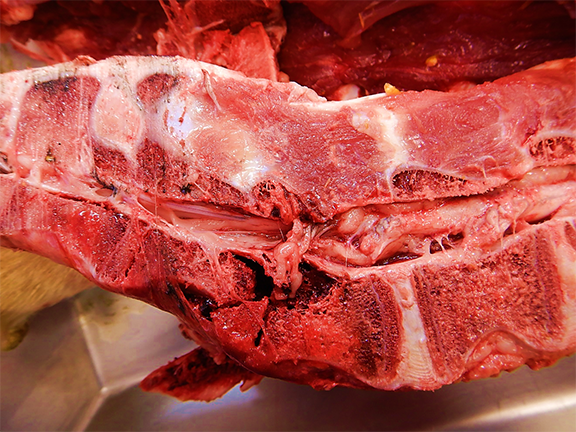
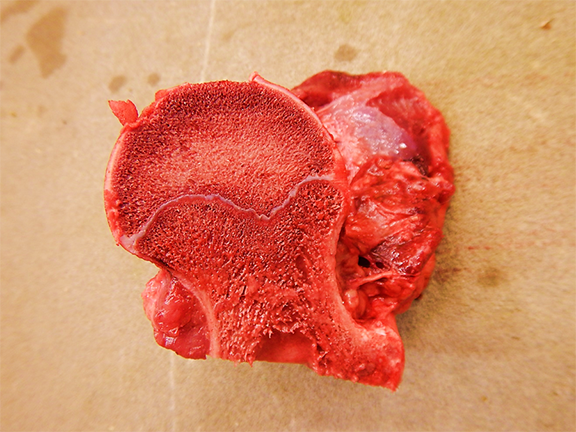
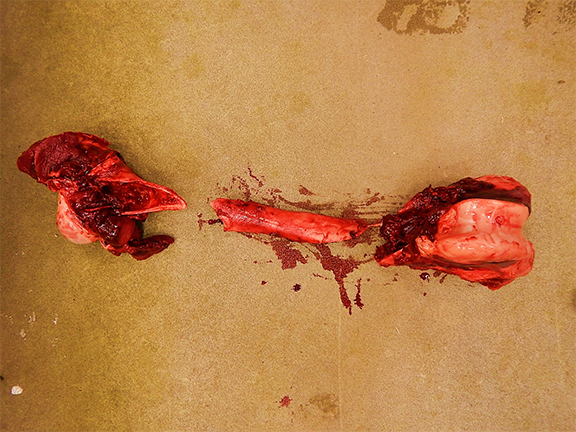
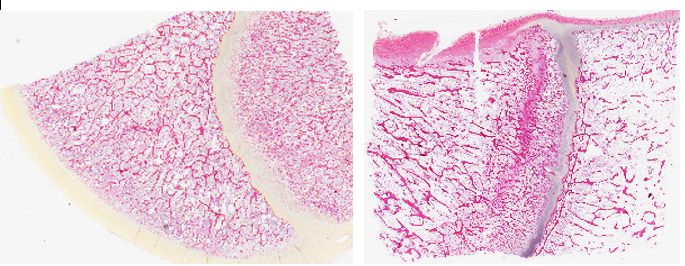
The necropsy of two calves from the affected group revealed multiple fractures, varying from an individual to another, involving the appendicular long bones, ribs, vertebrae and/or pelvis. Some fractures appeared old (bone callus on the ribs) but others were fresh and associated with severe hemorrhage in the surrounding soft tissues. The bones were firm, not rubbery, but were easier to cut than normal. The growth plate of the long bones was abnormally thin and the cancellous bone appeared less dense than expected. Notably, the decalcification process of long bones to make histological sections was significantly reduced and easier than normal. The kidneys of both animals were normal grossly and their parathyroids were not enlarged. Veal calf No 2 had soft feces.
Laboratory Results:
Bones from both calves were sent for analysis. Bone ash (mineral content of bone) and density were significantly decreased in Calf No 1 (bone ash 24% (normal 59-60%), density 1.13 g/ml (normal 1.29-1.35)). Bone ash was mildly decreased in Calf No 2 (53%) but density was normal. Calcium was mildly increased in both animals (39% and 39.4%; normal 37%) and phosphorus was minimally decreased (17.1% and 17%; normal 18.5%). Serum copper was evaluated in Calf No 2 and appeared decreased (10.0 µmol/L; normal 12-19 µmol/L). In the same animal, RT-PCR for Rotavirus type A was mildly positive (CT 30.07) and coprology revealed no intestinal parasites.
Microscopic Description:
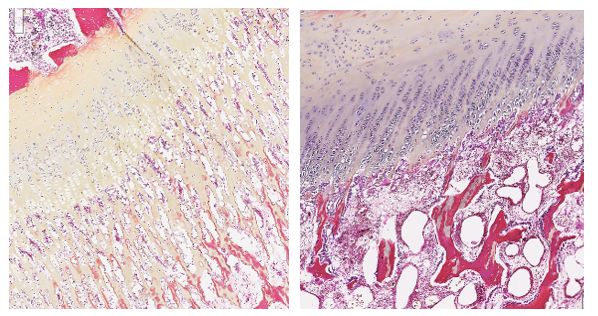
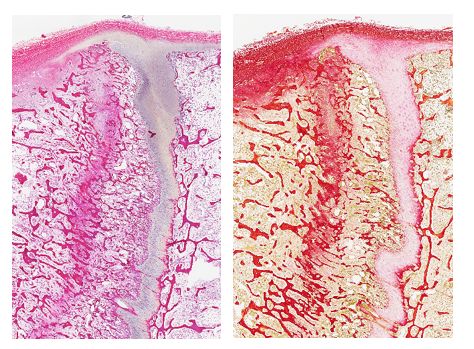
The examination of long bones revealed a decrease in thickness of the growth plate with variable narrowing of the hypertrophic zone and a multifocal loss of the secondary spongiosa and sometimes even of the primary spongiosa. Numerous osteoclasts were located between the chondroid trabeculae. Osteoblasts were hypertrophied and hyperplastic and lined the osteoid trabeculae of the metaphysis. There was a marked reduction of bone trabeculae abundance in the epiphysis and metaphysis but those still present had a normal thickness and appeared normally ossified. A line of fibrosis, parallel to the growth plate, was observed in the metaphysis and was associated with an older incomplete fracture and bone remodeling.
Other tissues showed no significant lesions. The intestinal mucosa was not inflamed and crypts were not hypertrophied. However, the apex of the intestinal villi could not be evaluated due to post-mortem desquamation of epithelial cells.
Contributor's Morphologic Diagnoses:
Femur, Calf No 1: Osteoporosis with incomplete/transverse metaphyseal fracture with fibrosis
Contributor's Comment:
Osteoporosis is characterized by loss of bone density with normal mineralization: the number of bone trabeculae is reduced but those that remain have a normal structure. However, this reduction in density decreases bone strength and affected animals are prone to pathological fractures, as was the case with those veal calves that developed such lesions without any significant trauma or excessive strength used for restraining. Osteoporosis should be differentiated from rachitism and osteomalacia, in which the osseous matrix is normal but its mineralization is deficient, and from fibrous osteodystrophy, characterized by excessive bone resorption, proliferation of fibrous tissue and formation of immature and poorly mineralized bone.6
Observed in humans and domestic/farm animals, osteoporosis has also been described in wild ruminants such as moose (Alces alces) in Sweden, similar to the condition in cattle/sheep with a secondary copper (Cu) deficiency and/or molybdenosis, and in a Mountain Sheep (Ovis canadensis) and another moose both with osteoporotic skull anomalies.3,6,10,11 Osteoporosis is also frequently observed in commercial high-producing laying hens.20
The major causes of osteoporosis are advanced age (senile osteoporosis), inactivity (disuse osteoporosis), corticosteroid treatment and nutrition. Other potential causes to consider include vitamin A toxicity, hyperthyroidism, chronic exposure to cadmium and the use of some immunosuppressive or anticonvulsant agents.6 In this particular case, all causes were not considered relevant with the exception of a potential nutritional problem, which was deemed the most likely etiology. Such a nutritional problem could be primary (unbalanced diet, starvation) or secondary (intestinal malabsorption/inflammatory bowel disease). Although in humans chronic pancreatitis has been linked to osteoporosis and increased risk of bone fractures, no abnormalities were noted in bone pliability/fragility in an 8-y-old lactating Holstein cow in spite of pancreatholithiasis, pancreas atrophy/fibrosis and a fractured lumbar vertebrae.14 In a case series of ten 2-y-old dairy heifers in first-lactation from New Zealand, humeral fractures were associated with osteoporosis, possibly linked to insufficient bone deposition during growth following protein-caloric malnutrition.7 A short communication details osteoporosis in a group of 15 goats from Switzerland in which the cause was a combined calcium/phosphorus and vitamin D deficiency as well as malnutrition attributed to gastrointestinal parasitism.5
Veal calves from our case were in excellent body condition so their diet seemed sufficient to fill their energy requirements. Even if the second calf had soft feces and a mildly positive RT-PCR for Rotavirus type A, these two animals showed no significant inflammation and/or atrophy of the intestinal villi and coprology revealed no intestinal parasitism. All other tissues were unremarkable grossly and microscopically. As such, a dietary imbalance was considered the most likely hypothesis in the present case. Calcium, phosphorus and copper deficiency could cause osteoporosis. In ruminants, calcium deficiency is often associated with this type of lesion, even if in other species this imbalance mostly results in fibrous osteodystrophy.6 Parathyroid activity and size increase when the serum calcium is low, leading to bone resorption; however, in the present case, parathyroids appeared normal grossly and calcium levels were mildly elevated in the bone ash of our two animals (39% and 39.4%, respectively).
Apart from this elevated calcium level and a reduced bone density, bone ash analysis demonstrated a reduction in phosphorus level in the mineral matrix (17% and 17.1% in animals Nos 1 and 2, respectively). According to Puls, phosphorus bone level below 17.6% could be considered critical for the health of an animal.18 However, bone ash analysis must be interpreted with caution as the reference intervals for calcium and phosphorus are not necessarily reliable and because there would be no significant changes in most osteoporosis cases due to the stable calcium: phosphorus ratio in hydroxyapatite crystals that form the mineral matrix. But if we still consider this decreased phosphorus level as significant, it is noteworthy that chronic phosphorus deficiency usually induces rickets or osteomalacia rather than osteoporosis. Current scientific literature is not clear on the factors that could trigger bone to develop osteoporosis instead of rickets when phosphorus levels are low.6 Interactions with other minerals could explain these differences. Copper, molybdenum and manganese have all been reported to be involved in some cases of osteoporosis. Bone copper was not assessed in the present cases but serum copper was evaluated in the second animal and was slightly decreased. Since we have very few data to determine the normal serum copper values in bovine and because this result reflects the copper level at one specific time point in a process that is most likely subacute to chronic, this mild serum copper decrease should be interpreted with caution. Unfortunately, we had no follow up from the referring veterinarian concerning this herd problem and its origin remains obscure.
Osteoporosis in humans is a chronic systemic bone disease of growing relevance due to the on-going demographic change in our aging societies. As defined by the world Health Organization (WHO), osteoporosis corresponds to a bone mineral density (BMD) T-score less than -2.5 SD as measured by dual-emission x-ray absorptiometry (DXA), commonly affecting 30% of women and 12% of men at some point during life.1 The annual costs in the US of caring for osteoporotic-related fractures parallel or exceed the annual costs of caring for myocardial infarction, breast cancer and/or cerebrovascular accident.15 In humans, risk factors associated with osteoporotic fractures include low peak bone mass, hormonal factors, use of certain drugs, cigarette smoking, low physical activity, low calcium/vitamin D intake, race, small body size and a personal/family history of fractures.5,8
As in humans, glucocorticoid-induced postmenopausal/ovariectomy-related osteoporosis in lab animals is a significant form of osteoporosis, with higher risk of osteoporotic fractures.21 Many different animal species have been used as models of that bone disorder for research. Although rodents are attractive to that effect because of the possibility of specifically modifying their genetic background, some aspect of that research can only be addressed with large animal models, such as metaphyseal fracture healing. Among large animal species, sheep have proven invaluable for osteoporosis research in this context.17 Different ewe models for osteoporosis have been successfully established and are very important for orthopedic research, such as the lumbar spine vertebral compression fracture in a sheep osteoporosis model.9,16 In the context of osteoimmunology, TNF-α and IL-6 are considered potential candidates for clinical application in spite of contradictory roles in immune responses versus bone metabolism.19 Another interesting research area involves an osteoporosis model using the red deer (Cervus elaphus) stag, in which physiological osteoporosis is a consequence of the annual antler cycle. This phenomenon could allow the identification of genes involved in the regulation of bone mineral density using comparative genomics between human and deer.4
Contributing Institution:
Centre de Diagnostic Vétérinaire de l'Université de Montréal
Faculté de médecine vétérinaire
Université de Montréal
3220 Sicotte
Saint-Hyacinthe,
Québec, CANADA
J2S 2M2
https://fmv.umontreal.ca/services/centre-de-diagnostic-veterinaire-de-luniversite-de-montreal/
JPC Diagnosis:
Long bone with growth plate: Osteopenia, diffuse, marked with metaphyseal infraction and fibrotic endosteal callus.
JPC Comment:
The contributor provides an excellent review of multiple underlying pathogeneses associated with the development of osteoporosis, a condition associated with increased bone fragility. Osteoporosis occurs as a result of excessive bone resorption in comparison to deposition, with a reduced quantity of bone while normal bone quality is retained.
As noted by the contributor, osteoporosis commonly affects postmenopausal women as well as older men, posing a significant risk factor for the development of fragility fractures, which most commonly involve the spine and metaphyseal bone in the hips and wrist. Furthermore, the features of osteoporosis may inherently complicate stabilization efforts (e.g. surgical fixation) necessary for optimal bone apposition, leading to unfavorable mechanical conditions and prolonged healing.2
Historically, rodent models have predominantly been used in ovariectomy-induced osteoporosis fracture studies, as well as to identify genes involved in fracture healing of osteoporotic bones. Although these species are ideal for use in these types of studies, their small size makes them less than ideal animal models for other related studies, such as those assessing orthopedic implants or in depth analysis of metaphyseal fracture healing. As noted by the contributor, sheep have been utilized as an animal model for these types of studies, with experimental induction traditionally via ovariectomy and/or glucocorticoid administration. Compared to ovariectomy, which only causes marginal osteoporosis in sheep, significant bone loss is induced by glucorticoid administration. Unfortunately, the dosage of glucocorticoids required to induce osteoporosis raises animal welfare concerns due to undesirable side effects. An alternative method, surgical disconnection of the pituitary gland from the hypothalamus, has been found to induce osteoporosis in sheep, with considerable bone loss after 8 months. Disruption of the hypothalamo-pituitary axis inhibits leptin signaling, which plays a key role in bone regulation, while also altering levels of other hormones that regulate bone homeostasis such as gonadotropins, thyroid hormones, insulin-like growth factor 1, and cortisol. The only clinically symptoms reported to be associated with hypothalamic-pituitary disconnection in sheep are polydipsia and polyuria as a result of iatrogenic diabetes insipidus.2
The most common pharmaceutical class used to treat for osteoporosis in humans are bisphosphonates, which have been in clinical use since 1968. These inorganic pyrophosphate analogs have a high affinity for calcium and accumulate in the skeleton, becoming embedded in newly formed bone during the anabolic phase of remodeling. Following their deposition, these substances remain inert until they're released in the acidic Howship's lacuna and absorbed by osteoclasts. Following their absorption, bisphosphonates induce osteoclast apoptosis, resulting in an overall suppression bone resorption.12
Bisphosphonates have a long half-life (up to 10 years) due to their incorporation into bone. This is significant in that bone turnover can continue to be suppressed long after administration is discontinued. While this feature is beneficial in regard the treatment of osteoporosis, it may also associated with impairment of fracture repair. Although these medications do not appear to be detrimental to initial callus formation following a fracture, studies in multiple species have found they hinder the callus' remodeling due to the same mechanisms as previously described, resulting in delayed the conversion of woven bone to mature lamellar bone. Therefore a history of bisphosphonate administration may be considered a risk factor for delayed union. However, human studies have also found de novo bisphosphonate administration prescribed as the result of an osteoporosis related fracture is not associated with delayed healing in human patients, likely due to insufficient accumulation of the bisphosphonates in the skeleton. Furthermore, other studies have paradoxically found bisphosphonates may also accelerate fracture healing, such in rabbits with mandibular fractures treated with zoledronic acid.12
References:
1. Armas LAG, Recker RR. Pathophysiology of Osteoporosis: New Mechanistic insights. Endocrinol Metab Clin N Am. 2012;41:475-486.
2. Bindl R, Oheim R, Pogoda P, et al. Metaphyseal fracture healing in a sheep model of low turnover osteoporosis induced by hypothalamic-pituitary disconnection (HPD). J Orthop Res. 2013;31(11):1851-1857.
3. Bleich VC, Stahmann JG, Bowyer RT, Blake, JE. Osteoporosis and cranial asymmetry in a Mountain Sheep (Ovis canadensis). Journal of Wildlife Diseases. 1990;26(3):372-376.
4. Borsy A, Podani J, Stéger V, et al. Identifying novel genes involved in both deer physiological and human pathological osteoporosis. Mol Genet Genomics. 2009;281:301-313.
5. Braun U, Ohlerth S, Liesegang, A, et al. Osteoporosis in goats associated with phosphorus and calcium deficiency. Vet Rec. 2009;164:211-213.
6. Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie, G ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Elsevier; 2016
7. Dittmer KE, Hitchcock B, McDougall S, Hunnam JC. Pathophysiology of humeral fractures in a sample of dairy heifers. N Z Vet J. 2016;64(4):230-237.
8. Dontas IA, Yiannakopoulos CK. Risks factors and prevention of osteoporosis-related fractures. J. Musculoskelet Neuronal Interact. 2007;7(3) :268-272.
9. Eschler A, Röpenack P, Herlyn PKE, et al. The standardized creation of a lumbar spine vertebral compression fracture in a sheep osteoporosis model induced by ovariectomy, corticosteroid therapy and calcium/phosphorus/vitamin D-deficient diet. Injury, Int. J. Care Injured. 2015;46S4:S17-S23.
10. Frank A. Mysterious Moose disease in Sweden. Similarities to copper deficiency and/or molybdenosis in cattle and sheep. Biochemical background of clinical signs and organ lesions. Science Total Environment. 1998;209:17-26.
11. Hindelang M, Peterson RO. Osteoporotic skull lesions in Moose at Isle Royale National Park. Journal of Wildlife Diseases. 1996;32(1):105-108.
12. Kates SL, Ackert-Bicknell CL. How do bisphosphonates affect fracture healing?. Injury. 2016;47 Suppl 1(0 1):S65-S68
13. Lane NE. Epidemiology, etiology and diagnosis of osteoporosis. American Journal of Obstetrics and Gynecology. 2006;194:S3-11.
14. Lopez A, McKenna S. Bovine pancreatolithiasis: case report and review of the literature. Journal of Veterinary Diagnostic Investigation. 2018;30(5):760-762.
15. Miller PD. Management of severe osteoporosis. Expert Opinion of Pharmacotherapy. 2016;17(4):473-488.
16. Oheim R, Amling M, Ignatius A, Pogoda P. Large animal model for osteoporosis in humans: The ewe. European Cells and Materials. 2012;24:372-385.
17. Oheim R, Schinke T, Amling M, Pogoda P. Can we induce osteoporosis in animals comparable to the human situation? Injury, Int. J. Care Injured. 2016;47S1:S3-S9.
18. Puls R. Mineral Levels in Animal Health: diagnostic data. 2nd ed. Sherpa international;1994.
19. Wang T, He C. TNF-α and IL-6: The link between immune and bone system. Curr Drug Targets. 2020;21(3):213-227.
20. Webster AB. Welfare implications of Avian Osteoporosis. Poultry Science. 2004;83:184-192.
21. Zhang Z, Ren H, Shen G, et al. Animal models for glucocorticoid-induced postmenopausal osteoporosis: An updated review. Biomedicine & Pharmacotherapy. 2016;84:438-446.