Signalment:
Histopathologic Description:
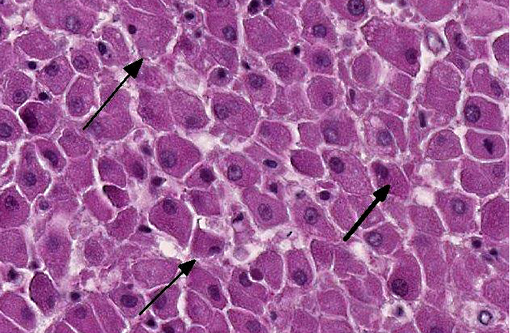
In portal tracts, bile ducts are distended by an amphophilic mucinous substance (moderate bile duct mucostasis). The wall of centrolobular veins is infiltrated by a few lymphocytes and neutrophils (discrete lymphocytic and neutrophilic transparietal vasculitis).
Etiology: Leptospira interrogans serovar icterohaemorrhagiae.
Lab Results:
Condition:
Contributor Comment:
Clinical signs associated with leptospirosis vary and depend on the serovar and the host. In maintenance hosts, leptospirosis generally is characterized by a low serological response, relatively mild acute clinical signs, and a prolonged renal carrier state which may be associated with chronic renal disease. In incidental hosts, leptospirosis can cause severe disease, associated with high titers of agglutinating antibody, and has a short or negligible renal carrier state. The clinical signs observed vary with the susceptibility of the host and with the infecting serovar. In general, young animals are more seriously affected than adults.(1,2) Clinical signs reported in dogs with leptospirosis include fever, inappetence, vomiting, abdominal pain, diarrhea, polyuria/polydipsia, myalgia, jaundice, epistaxis, hematuria and reproductive failure.(1,2) Signs of hepatic and renal dysfunction and of coagulation defects usually predominate in dogs with leptospirosis. The severity of clinical signs depends on the age and immunocompetence of the host, the environmental factors affecting the organisms, the serovar involved and its virulence, and the infecting dose. Younger dogs (less than six months) are more severely affected and develop more signs of hepatic dysfunction. A majority of leptospiral infections in dogs are subclinical. The peracute form is characterized by massive leptospiremia, causing shock and often death with few premonitory signs. Less severe infections are characterized by fever, anorexia, vomiting, dehydration, increased thirst and reluctance to move.(3)
Renal colonization occurs in most infected animals because the organism replicates and persists in renal tubular epithelial cells, even in the presence of neutralizing antibodies. Acute impairment of renal function may result from decreased glomerular filtration caused by swelling that impairs renal perfusion. Renal function in some dogs that survive acute infections may return to normal within several weeks, or chronic compensated polyuric renal failure may develop.(3)
The liver is another major organ damaged during leptospirosis. The degree of icterus in both canine and human leptospirosis usually corresponds to the severity of hepatic necrosis. In contrast, the icterus, hemoglobinemia and hemoglobinuria which develop in cattle with leptospirosis result from a specific hemolytic toxin produced by serovar pomona.Â
Tissue edema and disseminated intravascular coagulation may occur rapidly and result in acute endothelial injury and hemorrhagic manifestations. Leptospira lipopolysaccharides stimulate neutrophil adherence and platelet activation, which may be involved in inflammatory and coagulatory abnormalities.(3) Benign meningitis is produced when leptospires invade the CNS; however, it is not as common as in humans. Uveitis is occasionally present. Abortion and infertility resulting from transplacental transmission of leptospires associated with serovar bataviae infection have been described. Pulmonary manifestations include labored respiration and coughing. Interstitial pneumonia has been documented as the cause in humans, whereas lung changes in dogs with leptospirosis are associated with pulmonary hemorrhage most likely due to endothelial damage and vasculitis.(3)
Laboratory abnormalities usually include leukocytosis, thrombocytopenia, increased serum urea and creatinine, electrolyte disturbances, bilirubinemia and increased serum hepatic enzyme activities. Coagulation parameters may be altered in severely affected animals. Urinalysis abnormalities include bilirubinuria, sometimes glucosuria, proteinuria and increased numbers of granular casts, leukocytes and erythrocytes in the sediment.(3)
Establishment of a diagnosis is important because animals can serve as reservoirs and pose potential zoonotic risks.(1)
The diagnosis of leptospirosis can be accomplished by several techniques. Detection of antibodies using the microscopic agglutination test (MAT) is the most common diagnostic method; however, other methods to detect antibodies, such as immune-� fluorescent assays or enzyme-linked immunosorbent assay (ELISA), have been used.(2,3) Application of silver stains, or immunohistochemical stains to tissue sections are effective for detection of leptospires or leptospiral antigens in the renal tubules and interstitium of the kidney and liver. Low sensitivity is a disadvantage of this diagnostic technique. Leptospires are often present in small numbers in affected tissues, particularly in chronic leptospirosis or in dogs treated with antibiotics. Serological studies should also be conducted.(2) Bacteriologic culture of blood, urine, or tissue specimens is the definitive method for the diagnosis of leptospirosis. Leptospiremia occurs early in the clinical course of leptospirosis and is usually of short duration and low level. Therefore, blood is only useful for culture in the first few days of clinical illness and before antibiotic therapy. Leptospires are usually present in the urine of animals with leptospirosis four to ten days after the onset of clinical signs. Culture of leptospires is difficult, time-consuming and requires specialized culture medium. However, isolation of the organism allows definitive identification of the infecting server.(2)
The spectrum of human disease caused by leptospires is extremely wide, ranging from subclinical infection to a severe syndrome of multi-organ infection with high mortality.(4) Leptospirosis is presumed to be the most widespread zoonosis in the world. The source of infection in humans is usually either direct or indirect contact with the urine of an infected animal. The incidence is significantly higher in warm-climate countries than in temperate regions; this is due mainly to longer survival of leptospires in the environment in warm, humid conditions.(4)
The clinical presentation of leptospirosis is biphasic, with the acute or septicemic phase lasting about a week, followed by the immune phase, characterized by antibody production and excretion of leptospires in the urine. Most of the complications of leptospirosis are associated with localization of leptospires within the tissues during the immune phase and thus occur during the second week of the illness.(4) A comparison of the prevalence of anti-leptospiral antibodies with the prevalence of clinical signs indicates that subclinical infections are relatively common.(2)
JPC Diagnosis:
Conference Comment:
Conference participants discussed the serovars of Leptospira interrogans that commonly affect domestic animals and humans. The included chart summarizes these serovars and their hosts (both maintenance and incidental), as well as associated clinical conditions:(4)
| Serovar | Maintenance host | Incidental host | Clinical conditions |
| Bratislava | Pigs, hedgehogs, horses | Dogs | Reproductive failure, abortions, stillbirths |
| Canicola | Dogs | Pigs, cattle | Acute nephritis in pups. Chronic renal disease in adult animals |
| Grippotyphosa | Rodents | Cattle, pigs, horses, dogs | Septicemic disease in young animals; abortion |
| Hardjo | Cattle, sheep, deer | Humans | Influenza-like illness; occasionally liver or kidney disease |
| Icterohaemorrhagiae | Rats | Domestic animals, humans | Acute septicemic disease in calves, piglets and lambs; abortions |
| Pomona | Pigs, cattle | Sheep, horses, dogs | Acute haemolytic disease in calves and lambs; abortions |
References:
2. Bolin CA. Diagnosis of leptospirosis: a reemerging disease of companion animals. Semin. Vet. Med. Surg. (Small Anim.) 1996;11:166- 171.
3. Hartmann K, Creene GE. Diseases caused by systemic bacterial infection. In: Textbook of Veterinary Internal Medicine. 6th ed. St. Louis, MO: Elsevier Saunders; 2005;616- 619.Â
4. Levett P. Leptospirosis. Clin. Microbiol. Rev. 2001;14: 296- 326.
*Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, FitzPatrick ES. Veterinary Microbiology and Microbial Disease. Ames, IA: Wiley; 2011: Kindle Edition, location 13471 of 35051.Â