WSC 22-23
Conference 17
Case I:
Signalment:
1 day old, female, commercial lineage, Sus scrofa domesticus, swine.
History:
Our research group was contacted to investigate a disease that have been affecting neonates piglets in a sow farm located in West of Santa Catarina, Brazil. The farm has 330 sows, in an intensive system. In the period between May 2020 and January 2021, the farm manager have reported the birth of piglets with large and caudally rotated ears (“Dumbo-like piglets”), with weakness, apathy and in most cases, dyspnea. The morbidity rate was 3,4%, the mortality was 3% and the lethality was 86%. Most of the affected piglets was born from gilts (77%), and with a bith average of 4.8 “Dumbo-like piglets” per litter. The piglets died 1 to 5 days after birth. We received 14 piglets for routine diagnosis investigation.
Gross Pathology:
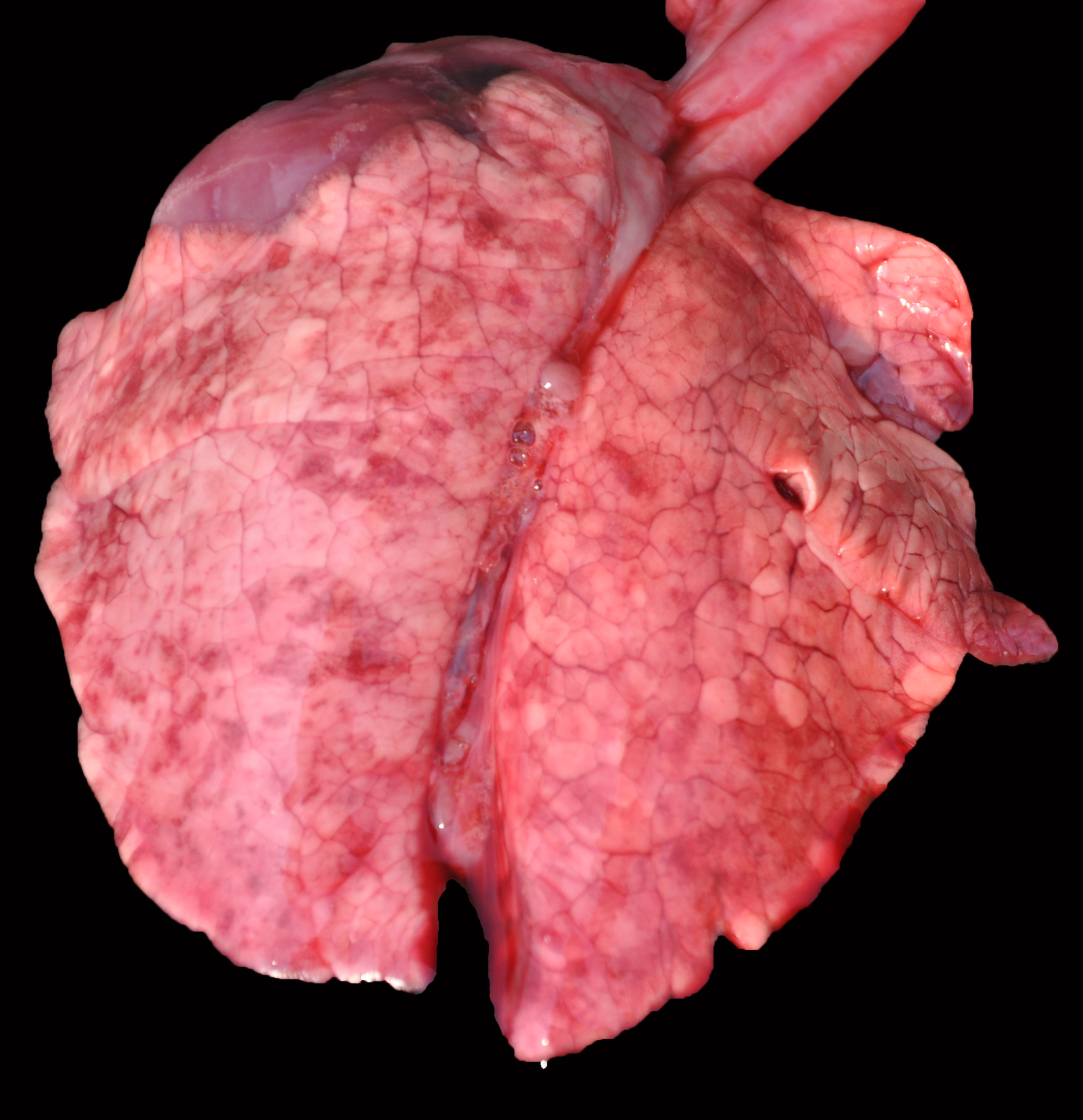
Grossly, the lungs of all piglets did not collapse upon the opening of the thoracic cavity, and also had marked interlobular edema. Also, all piglets had large and caudally rotated ears. No other significant abnormalities were visible in the remaining organs.
Laboratory Results:
PCR: The samples (pool of CNS, lung, heart, liver, and spleen) tested positive for PCV3 and negative for PCV1, PCV2, PPV 1, 2, 5, and 6, APPV, PRRSV, and OvHV-2.
ISH findings: Replication of PCV3 was most commonly observed in lymphocytes and plasma cells in perivascular areas and in the smooth muscle of arteries (fig. 3). We also observed PCV3 replication in lungs (fig. 4), heart (fig. 5), and in neurons of the brain.
Microscopic Description:
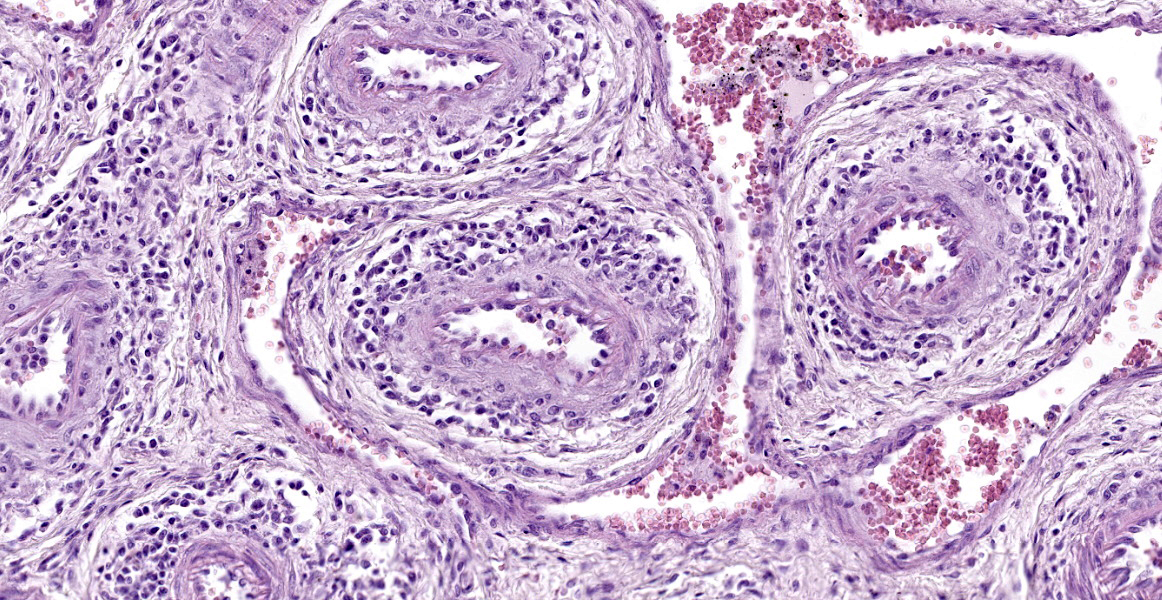
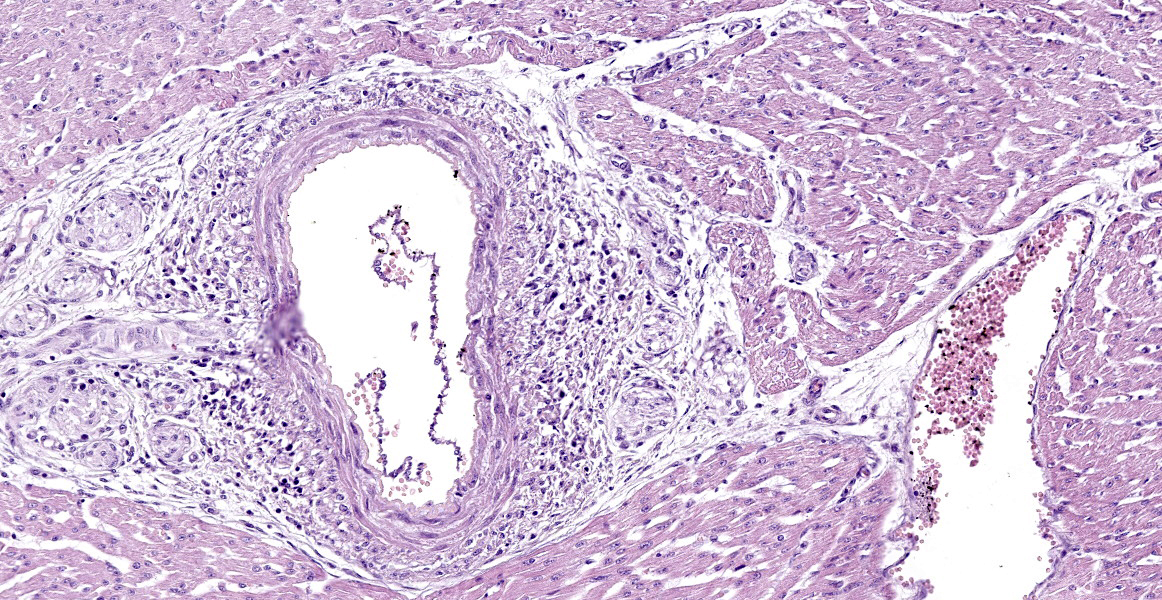
The slides submitted for this conference contained tissues from only one affected piglet. Vascular plexus and mesenteric lymph node: surrounding and disrupting the wall of vessels (mainly in arteries and arterioles), there is a severe and multifocal inflammatory infiltrate composed of lymphocytes, histiocytes and rare plasma cells. The infiltrate is associated with mild fibrinoid degeneration. In the mesenteric lymph node, mild necrosis of the germinal centers is observed, in addition to lymphoid rarefaction and mild infiltrate of macrophages containing brown pigment in their cytoplasm (hemosiderin).
Heart: in the pericardium and myocardium there is a moderate and multifocal inflammatory infiltrate surrounding and disrupting the vessel walls (mainly in arteries and arterioles), composed of lymphocytes, histiocytes, and rare plasma cells. There is also mild fibrinoid degeneration. Some cardiomyocytes present mild necrosis and are surrounded by lymphocytes, macrophages, and plasma cells.
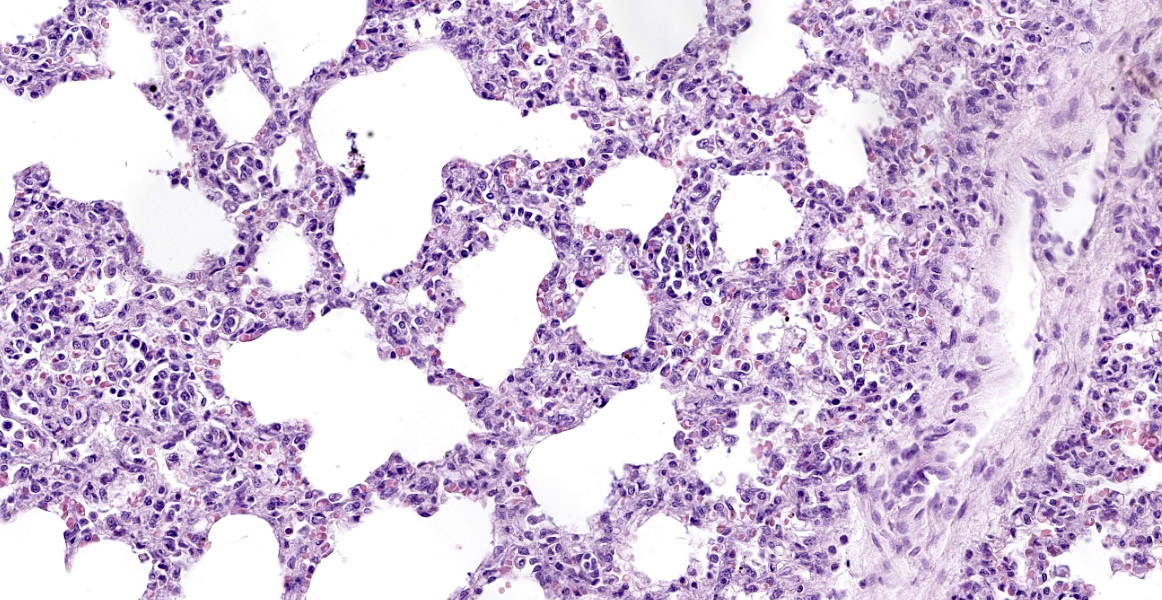
Lung: surrounding and disrupting the wall of vessels (mainly in arteries and arterioles), there is a severe and multifocal inflammatory infiltrate composed of lymphocytes, histiocytes, and rare plasma cells. A similar infiltrate is observed severely expanding the alveolar septa. There is also moderate and diffuse interlobular edema.
The other tissues presented a variable amount of lymphocytes, histiocytes, and plasma cells infiltrating and disrupting the wall of vessels, occasionally with fibrinoid degeneration, mainly in arteries and arterioles. Also, in the brain, there were multifocal areas of gliosis.
Contributor’s Morphologic Diagnoses:
Mesenteric plexus: lymphohistiocytic periarteritis and arteritis, multifocal, marked and mild fibrinoid degeneration;
Lymph node: lymphoid rarefaction and necrosis, multifocal, mild, and hemosiderosis, multifocal, mild.
Heart: lymphohistiocytic periarteritis and arteritis multifocal, marked and lymphohistiocytic myocarditis, multifocal, mild.
Lung: lymphohistiocytic interstitial pneumonia, diffuse, marked, with mild lymphohistiocytic periarteritis and arteritis.
Contributor’s Comment:
The diagnosis of PCV3-associated clinical disease in neonatal piglets was based on the molecular findings in association with the detection of PCV3 mRNA in microscopic lesions. PCV3 was first detected in 2015 in the United States, related to abortion cases, high mortality in sows, and clinical signs compatible with swine dermatitis and nephropathy syndrome.8 Also, PCV3 was described in three pigs from 3 to 9 weeks old, with multisystemic inflammation and myocarditis.9 Other clinical/pathological presentations were also associated with PCV3 infection, such as gastrointestinal, respiratory, and neurological disturbances.10 In most studies, the viral DNA has been detected in different tissues of pigs, however, without the demonstration of the agent in the lesions. PCV3 genetic material was detected through PCR in healthy piglet tissues,5, 8 which makes it difficult to conclude the real pathogenic potential of the virus and its economic impact on swine production. Therefore, the diagnosis of PCV3 must be confirmed through detecting its DNA in affected swine lesions, using techniques such as ISH, or at least associating the histopathological lesions with qPCR.
The ear malformation was a consistent feature observed in piglets of this farm. This finding was described in PCV3 positive pigs, previously,1, 6 however, it is necessary to perform more studies to characterize the pathogenesis of the large and caudally rotated ears and their relation to the PCV3 infection.
Multisystem vasculitis, myocarditis, and encephalitis were already PCV3-associated with the death of neonates,2, 6 and post-weaning piglets.9 Replication of PCV3 through ISH, was detected in the smooth muscle of arteries and arterioles.2 Lymphoplasmahistiocytic interstitial pneumonia was a constant pathological finding in the evaluated piglets, and through ISH it was possible to show the PCV3 viral replication within the lesions. This finding was described in a 19 day old piglet.9 Interstitial pneumonia is a common finding in viral infections of the inferior respiratory tract. In the maternity phase (usually until 21-25 days old), the main causes of viral pneumonia include the Aujeszky virus and PRRSV. There are no reports of clinical disease related to PRRSV presence in Brazil, and this virus was excluded through a PCR test. Pneumonia related to the Aujeszky virus can occur in piglets up until 5 days old; however, in this phase, neurologic signs are prominent, while respiratory signs are usually common in growing and finishing pigs.3 PCV2 is an important cause of interstitial pneumonia, described mainly in nursery and finishing piglets,4 and the lymphocytic and plasmacytic periarteritis is a common finding between PCV3 and PCV2 infection.2, 7 All samples tested negative for PCV1, PCV2, PRRSV, APPV, PPV, and OvHV-2, which are other potential causes of myocarditis, vasculitis, and encephalitis in neonatal piglets.
Contributing Institution:
Faculdade de Veterinária
Universidade Federal do Rio Grande do Sul
Setor de Patologia Veterinária
JPC Diagnosis:
- Lung: Pneumonia, interstitial, histiocytic, diffuse, severe, with intralobular edema and histiocytic arteritis.
- Mesenteric vascular plexus, heart: Arteritis, histiocytic, diffuse, moderate.
- Lymph node, subcapsular and medullary sinuses: Hemosiderosis, moderate.
JPC Comment: As the contributor states, porcine circovirus 3 was first diagnosed in 2015; however, retrospective studies have identified PCV-3 in samples from as early as the 1960s.7 And even though there is still much to be elucidated regarding this relatively young virus, PCV-3 is no longer the newest circovirus on the block: PCV-4 was discovered in 2019 in 7-week old pigs with respiratory disease, porcine dermatitis and nephropathy syndrome, and diarrhea.7
PCV-3 has been detected in a wide range of species and produces viremia in cattle, dogs, and laboratory mice.7 Wild boar are thought to be a reservoir, with prevalence of up to 61.54%.7 Mosquitos can also be infected, but studies have failed to support vector-borne transmission.7 In fact, the precise mode of transmission has not been worked out, and virus has been identified in oral fluids, the salivary glands, colostrum, semen, and testicles.7 The virus can infect a wide variety of cells and has been found in myocardiocytes, vascular smooth muscle, trophoblasts, adipocytes, ependymal cells. This suggests that the virus ligand binds to a widely expressed host receptor, which has yet to be identified.7 Affected pigs may have a persistent infection with prolonged viremia.7 Determining the effects of PCV-3 infection is challenging for multiple reasons: as the contributor states, there are a wide variety of syndromes associated with infection, but the virus has also been isolated in subclinical animals. Additionally, PCV-3 infection regularly occurs alongside other infectious agents, including PCV-2, porcine parvovirus, porcine reproductive and respiratory syndrome virus, making attribution of clinical signs with a specific agent difficult.7
A recent retrospective study reviewed formalin-fixed paraffin-embedded tissues from 587 pigs free of porcine circovirus 2 in Spain.4 PCV-3 ISH and qPCR were conducted on a subset of cases which contained lesions reported to be associated with PCV-3, including reproductive disease, congenital tremors, porcine dermatitis and nephropathy syndrome, arteritis or periarteritis, myocarditis, encephalitis, and peri-weaning failure-to-thrive syndrome. 4 All evaluated cases of periarteritis were qPCR positive for PCV3, and most were positive via IHC. 4 The most consistently affected sites for periarteritis were the mesenteric arteries and the kidney, indicating they are likely suitable choices for diagnostic testing. 4 Additionally, PCV-3 was not detected in cases of myocarditis and encephalitis where periarteritis was lacking. 4
The moderators and conference participants discussed a unique anatomic feature of pigs evident in this slide: the mesenteric arterial plexus. While the exact function is unknown, it may serve as a countercurrent mechanism to help regulate blood pressure. Conference participants also discussed the few foci of histiocytic infiltrates in the myocardium; the significance of these infiltrates is unknown.
References:
- Alomar J, Saporiti V, Pérez M, Gonçalvez D, Sibila M, Segalés J. Multisystemic lymphoplasmacytic inflammation associated with PCV-3 in wasting pigs. Transbound Emerg Diseases 2021; 1-6.
- Arruda B, Pineyro P, Derscheid R, Hause B, Byers E, Dion K, Long D, Sievers C, Tangen J, Williams T, Schwartz K. PCV3-associated disease in the United States swine herd. Emerg Microbes Infect 2019; 8:684-698.
- Ciacci-Zanella LR. Doença de Aujeszky. In: Megid J, Ribeiro MG, Paes AC (eds) Doenças infecciosas em animais de produçao e companhia, 2016, 1st ed. Roca, Rio de Janeiro, 598-602.
- Cobos A, Sibila M, Alomar J, Perez M, Huerta E, Segales J. Retrospective assessment of porcine circovirus 3 (PCV-3) in formalin-fixed paraffin-embedded tissues from pigs affected by different clinical-pathologic conditions. Porcine Health Manag. 2022; 8(1):51-60.
- França TN, Ribeiro CT, Cunha BM, Peixoto PV. Circovirose suína. Pesq Vet Bras 2005; 25(2):59-72.
- Franzo G, Delwart E, Fux R, Hause B, Su S, Zhou J, Segalés J. Genotyping porcine circovirus 3 (pcv-3) nowadays: does it make sense? Viruses 2020; 12(3):265.
- Kroeger M, Temeeyasen G, Pineyro PE. Five years of porcine circovirus 3: What have we learned about the clinical disease, immune pathogenesis, and diagnosis. Virus Res. 2022; 314: 198764.
- Molossi FA, Almeida BA, Cecco BS, Silva MS, Mósena ACS, Brandalise L, Simao GMR, Canal CW, Vanucci F, Pavarini SP, Driemeier D. A putative PCV3-associated disease in piglets from Southern Brazil. Braz J Microbiol 2022; 53:491-498.
- Opriessnig T, Janke BH, Halbur PG. Cardiovascular lesions in pigs naturally or experimentally infected with porcine circovirus type 2. J Comp Pathol 2006; 134:105-110.
- Palinski R, Pineyro P, Shang P, Yuan F, Guo R, Fang Y, Byers E, Hause BM. A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. Virol J 2017; 91(1):e01879-16.
- Phan TG, Giannitti F, Rossow S, Marthaler D, Knutson T, Li L, Deng X, Resende T, Vanucci FA, Delwart E. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol J 2016; 13(1):184.
- Zhai SL, Zhou X, Zhang H, Hause BM, Lin T, Liu R, Chen QL, Wei WK, Hong D, Wen XH, Li W, Wang D. Comparative epidemiology of porcine circovirus type 3 in pigs with different clinical presentations. Virol J 2017; 14(1):222.