Signalment:
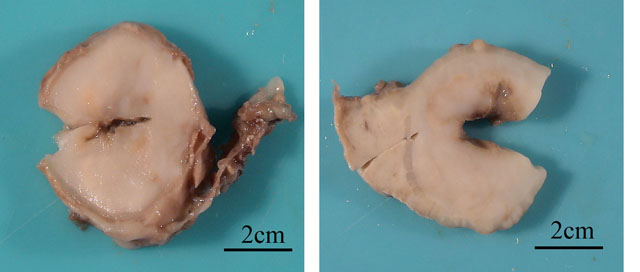
Gross Description:
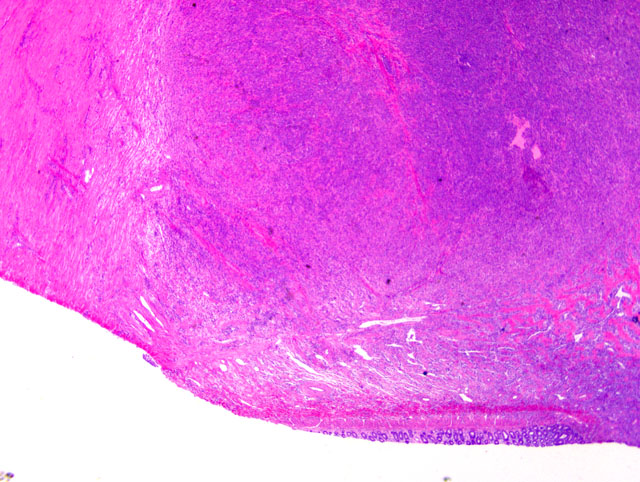
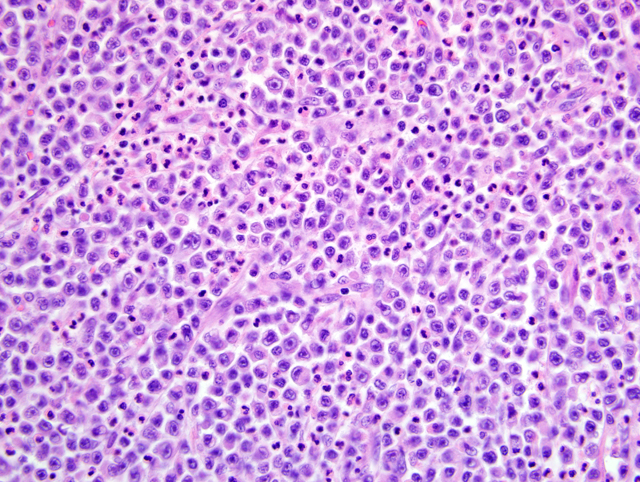
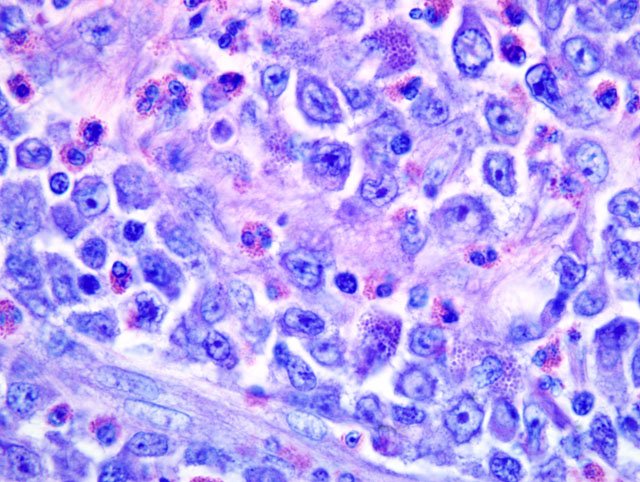
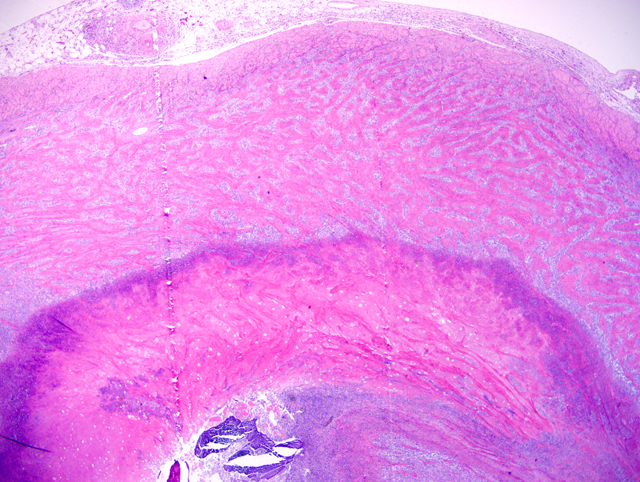
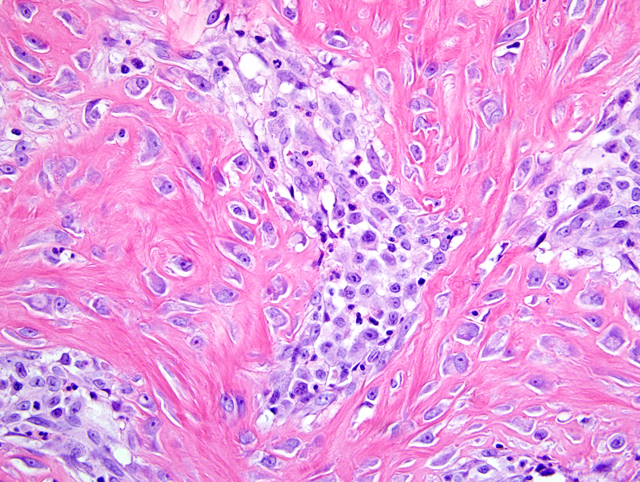
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Howl and Petersen (1995) described a single case report of an intestinal mast cell tumor in a cat that presented with eosinophilic enteritis. In addition to the marked eosinophilic infiltrate, this case was characterized by a marked scirrhous response surrounding the neoplastic population similar to the case presented here.(6) This demonstrates the diagnostic challenge the eosinophilic infiltrate may introduce. Thus, when confronted with a significant eosinophilic intestinal population, close attention to all histological features is necessary in order to differentiate feline intestinal sclerosing mast cell tumors from other lesions such as eosinophilic granulomatous disease(6) and feline gastrointestinal eosinophilic sclerosing fibroplasia.(2) While the marked eosinophilic infiltrate in these tumors may pose a diagnostic challenge, the presence of eosinophils is not uncommon with mast cell disease. Mast cells promote eosinophilic inflammation by producing eosinophil-directed cytokines, such as IL-4 and IL-5. These cytokines subsequently induce chemokines that specifically attract eosinophils, such as eotaxin-1 and 2.(9) In addition, the mucosal ulceration may be due, in part, to local vasoconstriction in response to the release of vasoactive substances from the mast cells, such as histamine, and subsequent devitalization of the overlying mucosal epithelium as well as by direct infiltration and mucosal effacement by the neoplastic cells.
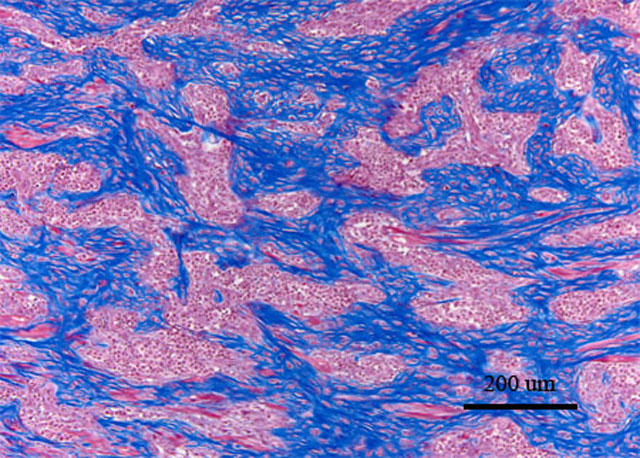
The sclerotic component was a unique feature in all cases evaluated. There is increasing evidence that mast cells play a central role in tissue remodeling in chronic inflammatory reactions. Among the numerous substances released by mast cell tumors are the fibrogenic cytokines fibroblast growth factor and transforming growth factor β1.(1,4) These cytokines promote activation, proliferation, and migration of fibroblasts with subsequent collagen production and contraction. In addition to mast cells, eosinophils have also been shown to play a role in fibrosis. Eosinophils are similarly involved in tissue remodeling and fibrosis in allergic reactions via the release of fibrogenic cytokines such as TGF-β and IL-1β.(2,4) The release of these fibrogenic cytokines by mast cells and/or eosinophils suggests a possible mechanism of mast cell-induced sclerosis in this tumor.
JPC Diagnosis:
1. Jejunum: Malignant round cell neoplasm.
2. Jejunum: Enteritis, ulcerative, eosinophilic and mastocytic, sclerosing, transmural, severe, with mixed bacteria and anisotropic foreign material.
Conference Comment:
The eosinophilic and sclerotic lesion in this case is remarkable, and the contributor offers a sound hypothesis for its pathogenesis. In a recent case series involving 25 cats, Craig and co-authors(2) proposed the term feline gastrointestinal eosinophilic sclerosing fibroplasia for a distinctive gastrointestinal lesion characterized by the mural presence of thick trabeculae of collagen separated by dense aggregates spindled cells. As in the present case, eosinophils and mast cells were numerous, and evenly scattered throughout the lesion. Immunohistochemical positivity for vimentin and smooth muscle actin was consistent with myofibroblastic differentiation and the authors speculated that bacteria were important in the pathogenesis of this condition. Additionally, the authors hypothesized that some cats may have a genetic predisposition to the development of this lesion, as has been proposed for other feline eosinophilic inflammatory conditions, in response to a variety of antigens. As a result, eosinophilic sclerosing fibroplasia may be thought of as a nonspecific reaction pattern to foreign antigen in genetically susceptible cats. In this case, the round cell neoplasm may have incited eosinophilic sclerosing fibroplasia by a number of mechanisms. Neoplastic cells, regardless of origin, may have directly recruited eosinophils and fibroblasts through cytokine production as hypothesized by the contributor. Alternatively, the neoplasm may have rendered the intestine vulnerable to antigen exposure by causing ulceration, partial obstruction, or altered peristalsis, allowing bacteria and digesta to penetrate the mucosa. This is supported by the presence of ulceration, numerous mixed bacteria, and embedded anisotropic foreign material (i.e. hair and food material) in many of the sections. We thank the contributor for this interesting submission.
References:
2. Craig LE, Hardam EE, Hertzke DM, Flatland B, Rohrbach BW, Moore RR: Feline gastrointestinal eosinophilic sclerosing fibroplasia. Vet Pathol 46:63-70, 2009
3. Fernandez NJ, West KH, Jackson ML, Kidney BA: Immunohistochemical and histochemical stains for differentiating canine cutaneous round cell tumors. Vet Pathol 42:437-435, 2005
4. Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ: Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immuno 116:796-804, 2005
5. Gross TL, Ihrke PJ, Walder EJ, Affolter VK: Feline mast cell tumors. In: Skin Diseases of the Dog and Cat, 2nd ed., pp. 858-861. Blackwell Publishing Co., 2005
6. Howl JH, Petersen MG: Intestinal mast cell tumor in a cat: presentation as eosinophilic enteritis. J Am Anim Hosp Assoc 31:457-461, 1995
7. Johnson TO, Schulman FY, Lipscomb TP, Yantis TP: Histopathology and biological behavior of pleomorphic cutaneous mast cell tumors in fifteen cats. Vet Pathol 39:452-457, 2002
8. Litster AL, Sorenmo KU: Characterisation of the signalment, clinical and survival characteristics of 41 cats with mast cell neoplasia. J Fel Med Surg 8:177-183, 2006
9. Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G: The role of human mast cell-derived cytokines in eosinophil biology. J Inter Cyto Res 24:271-281, 2004
10. Vail DM, Thamm DH: Mast cell tumors. In: Small Animal Clinical Oncology, eds. Withrow SJ, MacEwan EG, 4th ed., pp. 402-424. WB Saunders Co., 1997