Signalment:
Gross Description:
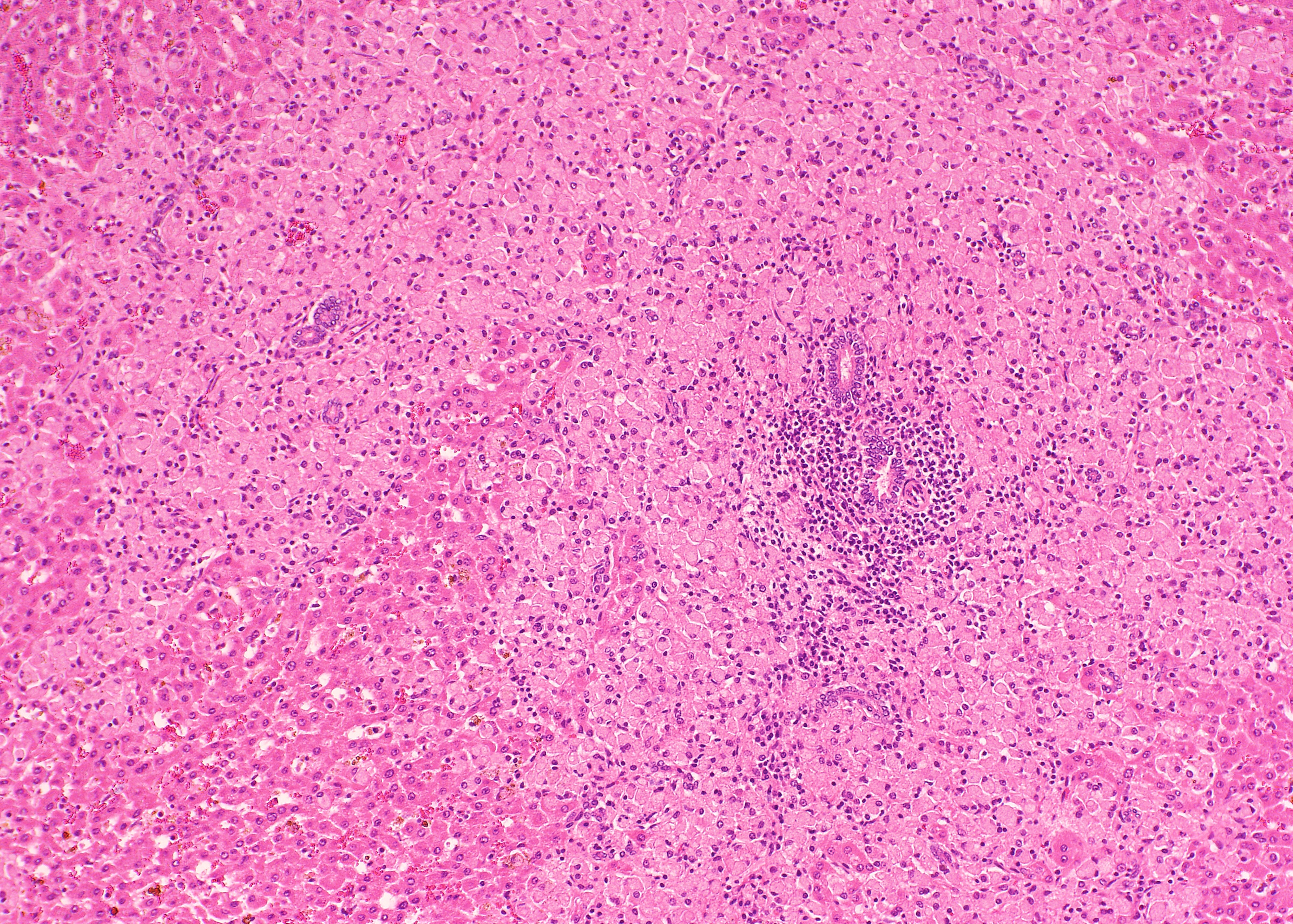
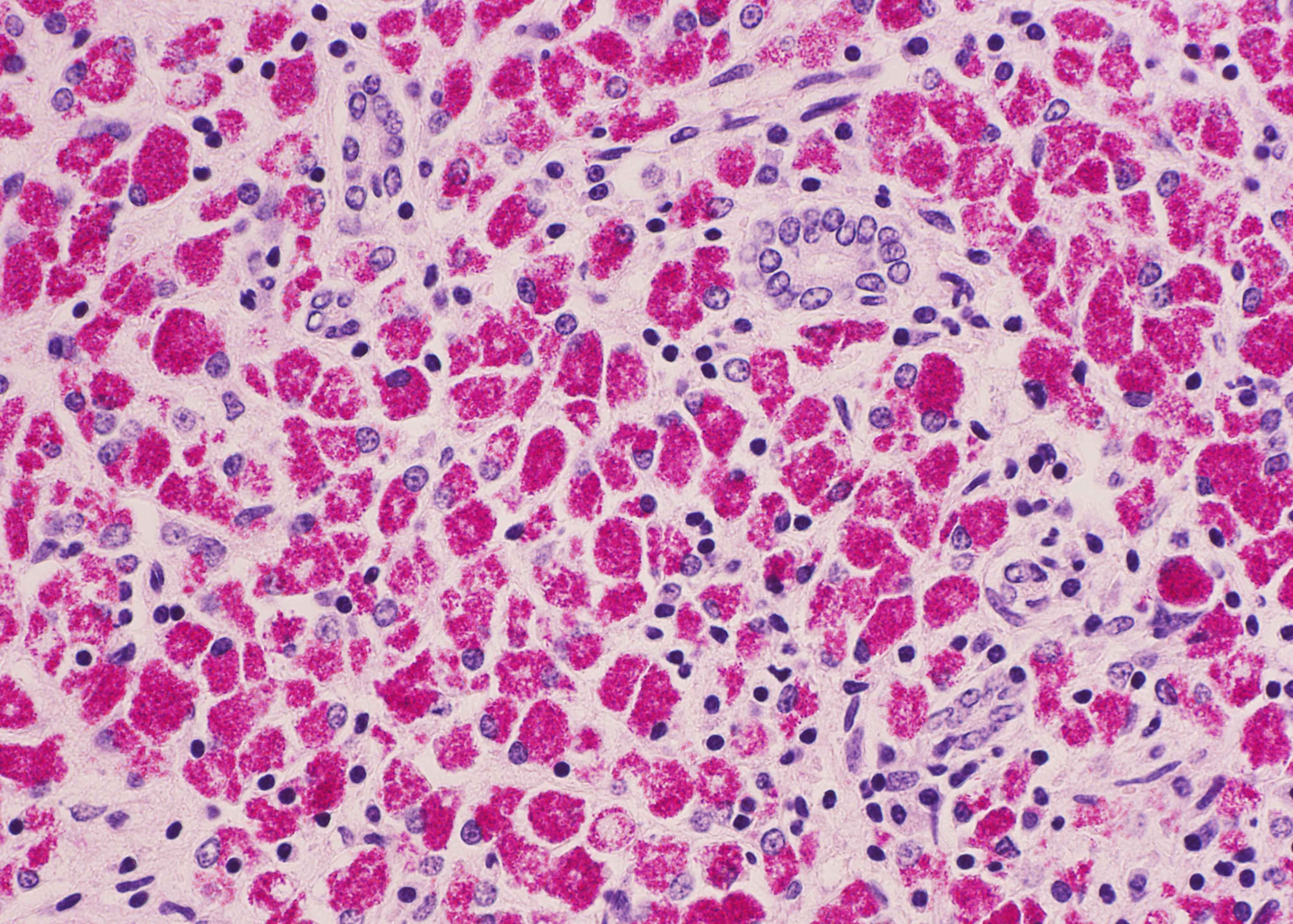
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
MAP is very resistant to environmental stressors, particularly in regions with acid soils. The bacterium enters the M cells that cover the intestinal Peyers patches, and ingested bacteria are subsequently phagocytized by macrophages.(2) In cattle, susceptibility to infection is greatest in the first 30 days of life, although clinical disease does not usually develop until 2-5 years of age. The progression from asymptomatic to clinical Johnes disease is associated with a decrease in peripheral cell-mediated immunity and increasing production of non-protective IgG1 antibodies.(7,8) The disease is clinically characterized by untreatable diarrhea accompanied by progressive weight loss and hypoproteinemia. The characteristic gross lesion in the symptomatic cattle is chronic segmental thickening of the ileum, mesenteric lymphadenopathy, and lymphangitis. Histologically, cattle usually have non-caseating granuloma in the affected bowel and lymphoid tissues.(2)
Although the pathogenesis of Johnes disease in small ruminants is assumed to be similar to that in cattle, there are several differences in clinical signs and pathology. Sheep and goats reveal emaciation and hypoproteinemia, but overt diarrhea is unusual.(2,5) In sheep, goats, and deer, enteric gross lesions are often mild, with little obvious thickening, and no transverse ridges.(2,4)
Two main pathological forms of the intestinal lesion have been described in symptomatic sheep.(3,9) The first form is multibacillary or lepromatous, in which macrophages filled with numerous mycobacteria are the main inflammatory cells. The second form is paucibacillary or tuberculoid, in which the inflammatory infiltrate is composed of lymphocytes and few macrophages and caseous necrosis and calcification may be observed. It is difficult to find acid-fast mycobacteria in macrophages in the latter form. It is uncertain whether the two distinct forms represent sequential or divergent stages. Asymptomatic sheep may have small granulomas in the interfollicular and basal areas of ileal Peyers patches, usually with no visible intracellular organisms.(9)
Corpa et al.(4) characterized the intestinal lesions of caprine tuberculosis in four categories: focal, diffuse multibacillary, diffuse lymphocytic, and diffuse mixed. Histological classification for sheep was valid for goats, and the lower frequency of focal lesions in goats compared to sheep appears to indicate that the former species has only limited ability to control the infection. In this species, granulomatous lesions are likely to be more severe in the jejunum than in the ileum although the distribution could not be found in this case. Currently, the similar histological classification is applicable also in bovine paratuberculosis.(6)
The ileum of the present case showed diffuse multibacillary lesions. The monitoring study in remaining sheep and goats supported the histological diagnosis. Previously, focal granulomas have been seen in lymph nodes elsewhere in the body, liver, lung, spleen, and other organs in symptomatic sheep and goats.(2-4) However, the amount of macrophagic infiltrates in the liver was unusual.
JPC Diagnosis:
Conference Comment:
Mycobacteria species utilize a complex interplay of virulence factors and the host immune system to evade being killed. The chart below, adapted from Weiss and Souza,(10) summarizes the mechanisms by which MAP suppresses monocyte-macrophage microbicidal response:
| Organism Factor | Cell Pathway | Mechanism | Result |
| Man-LAM | TLR2-MAPK-p38 | IL-10 overexpression | Decreased: IL-12, IL-8, and TNF-α, MHC class II, apoptosis, phagosome acidification, and organism killing |
| Man-LAM | IL-10-mediated | Decreased: IL-12, IL-8, and TNF-α expression | Attenuation of: 1. Inflammatory response 2. Th 1-type immune response |
| Man-LAM | IL-10-mediated | Decreased MHC class-II expression | Decreased antigen presentation |
| Unknown | IL-10-mediated; decreased TNF-α | Decreased apoptosis | Increased cell Survival |
| Man-LAM | IL-10-mediated; TLR2-MAPK-p38 signaling | Decreased phagolysosome fusion and acidification | Increased organism survival |
Interleukin 10, as noted in the chart above, plays a pivotal role in the MAP-directed immune modulation; a brief review of IL-10 follows. IL-10 is produced by a variety of cells, including T regulatory lymphocytes, Th 2 lymphocytes, and dendritic cells. The primary function of IL-10 is to direct the immune system to a Th 2-type immune response by enhancing Th 2 activity and suppressing Th 1 activity; Th 1 suppression is accomplished by inhibiting macrophage IL-12 production. Antigen presentation by dendritic cells is decreased through IL-10 mediated down-regulation of MHC class-II.(1)
As commented by the contributor, the distribution and extent of the histiocytic infiltrate in the liver is striking. While most participants interpreted the lesion as consistent with Mycobacterium avium subsp. paratuberculosis infection, they were nevertheless impressed by the number of infiltrating macrophages filled with acid- fast bacilli. A similar histopathologic presentation is reported in dogs infected with M. avium-intracellulare complex (MAC), as exemplified by Wednesday Slide Conference 2009 Conference 5 Case 1, and in the absence of special stains may result in the histologic interpretation of histiocytic neoplasia or a sarcomatous lesion, rather than an infectious process.
References:
2. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed., vol. 3. Philadelphia, PA: Elsevier Ltd; 2007:222-225.
3. Clark CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116:217-261, 1997
4. Corpa JM, Garrido J, Garc+�-�a Mar+�-�n JF, P+�-�rez. Classification of lesions observed in natural cases of paratuberculosis in goats. J Comp Pathol. 2000;122:255-265.
5. Gelberg HB. Alimentary system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. St. Louis, MO: Elsevier; 2007:372-374.
6. Gonz+�-�lez J, Geijo MV, Garc+�-�a-Pariente C, et al. Histopathological classification of lesions associated with natural paratuberculosis infection in cattle. J Comp Pathol. 2005;133:184-196.
7. Koets A, Rutten V, Hoek A, et al. Progressive bovine paratuberculosis is associated with local loss of CD4+ T cells, increased frequency of γδ T cells, and related changes in T-cell function. Infect Immune. 2002;70:3856-3864.
8. Kurade NP, Tripathi BN, Rajukumar K, Parihar NS. Sequential development of histologic lesions and their relationship with bacterial isolation, fecal shedding, and immune responses during progressive stages of experimental infection of lambs with Mycobacterium avium subsp. paratuberculosis. Vet Pathol. 2004;41:378-387.
9. P+�-�rez V, Gac+�-�a Mar+�-�n JF, Badiola JJ. Description and classification of different types of lesions associated with natural paratuberculosis infection in sheep. J Comp Pathol. 1996;114:107-122.
10.Weiss DJ, Souza CD. Modulation of mononuclear phagocyte function by Mycobacterium avium subsp. paratuberculosis. Vet Pathol. 2008;45:829-841.