Signalment:
Gross Description:
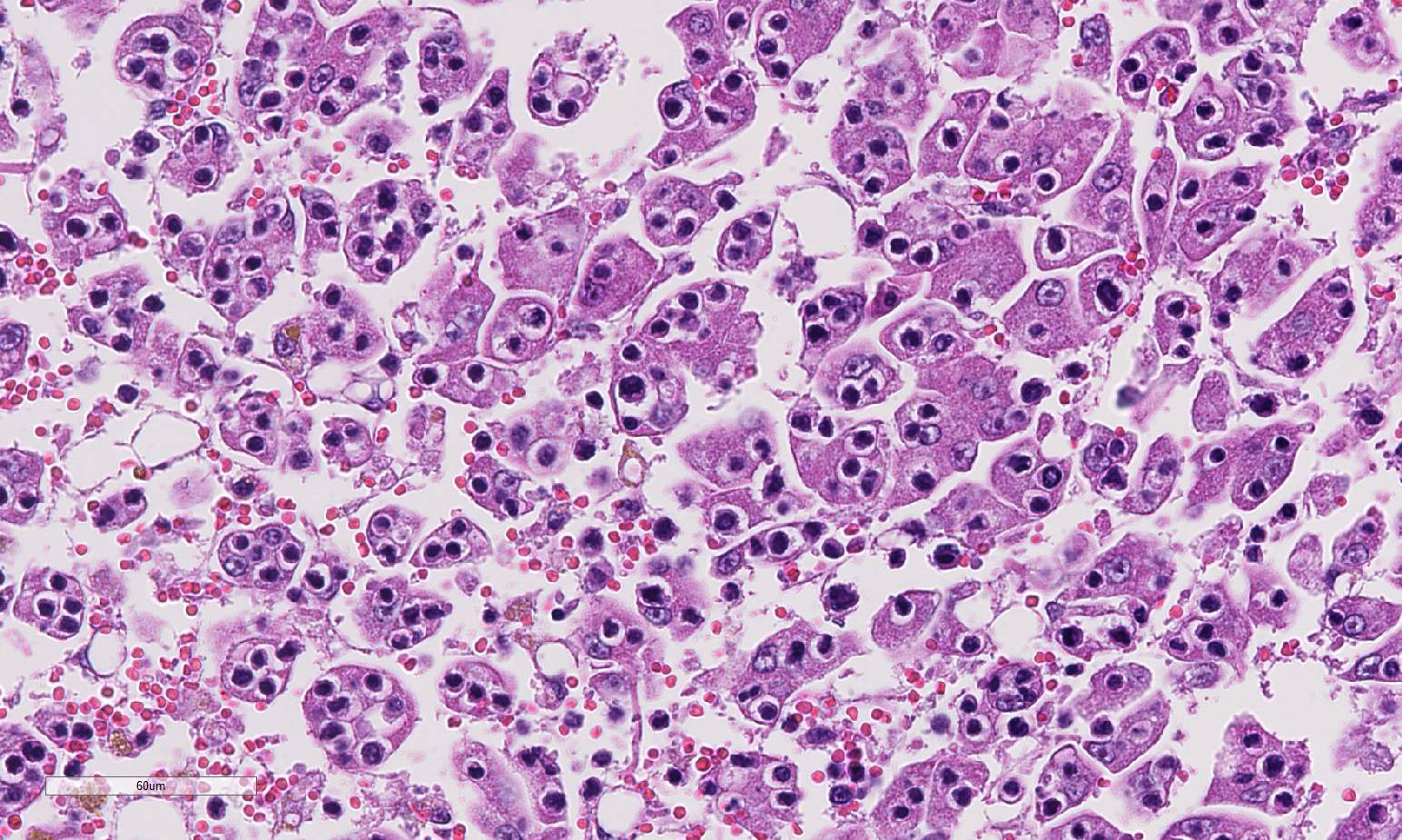
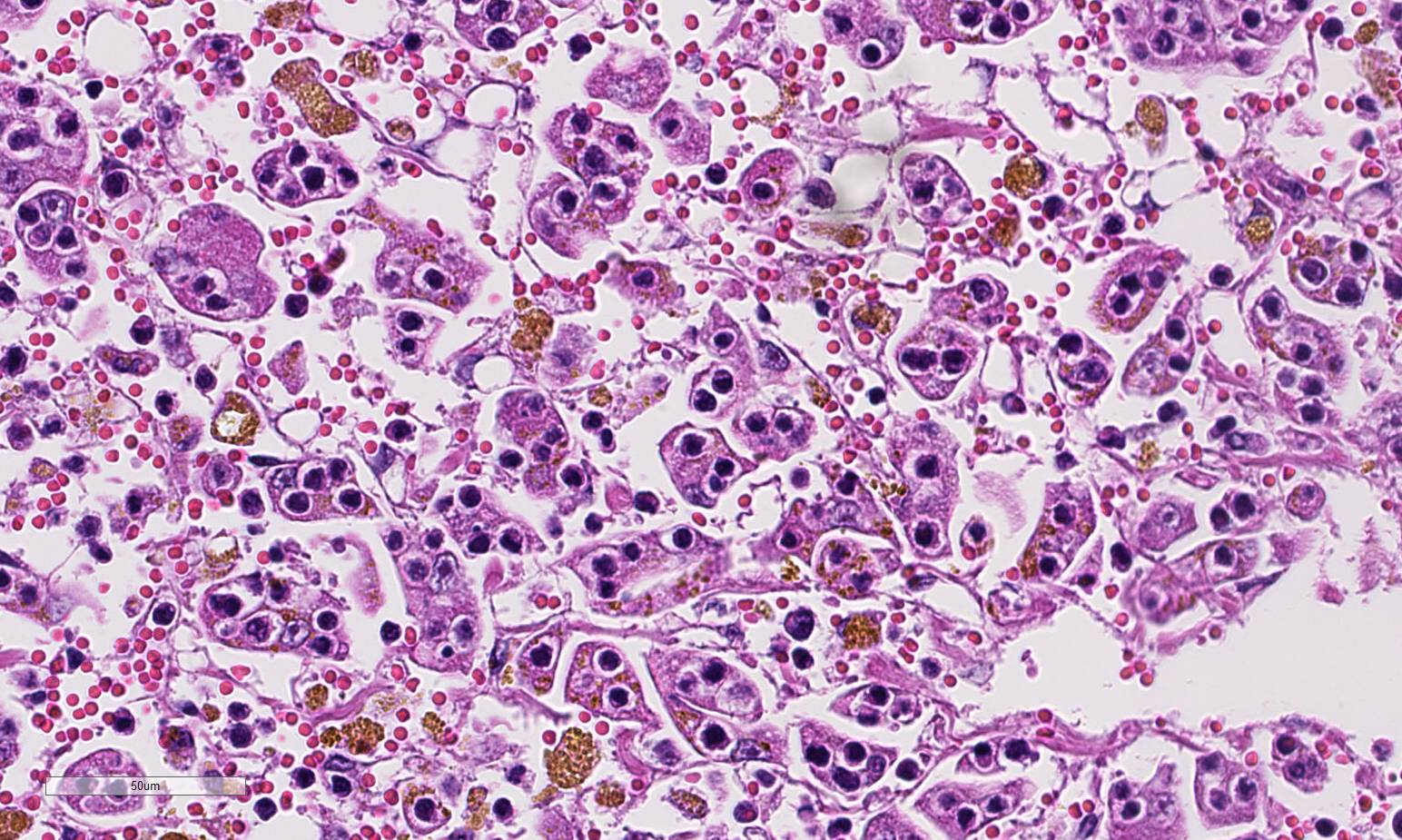
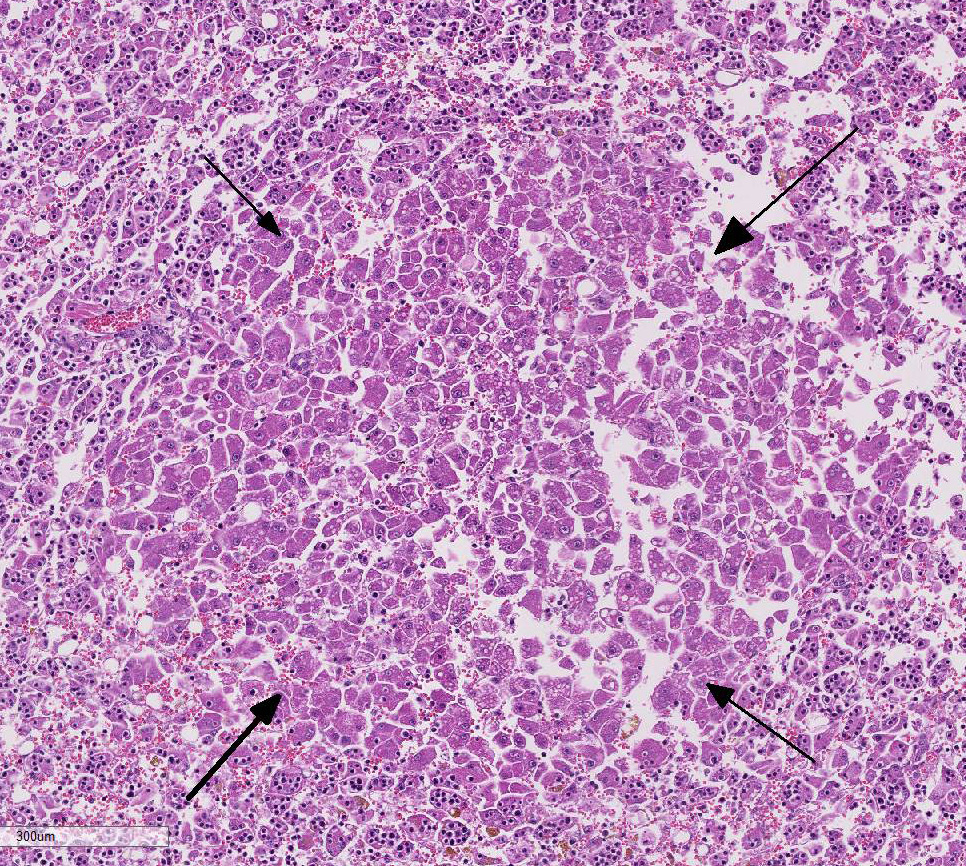
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
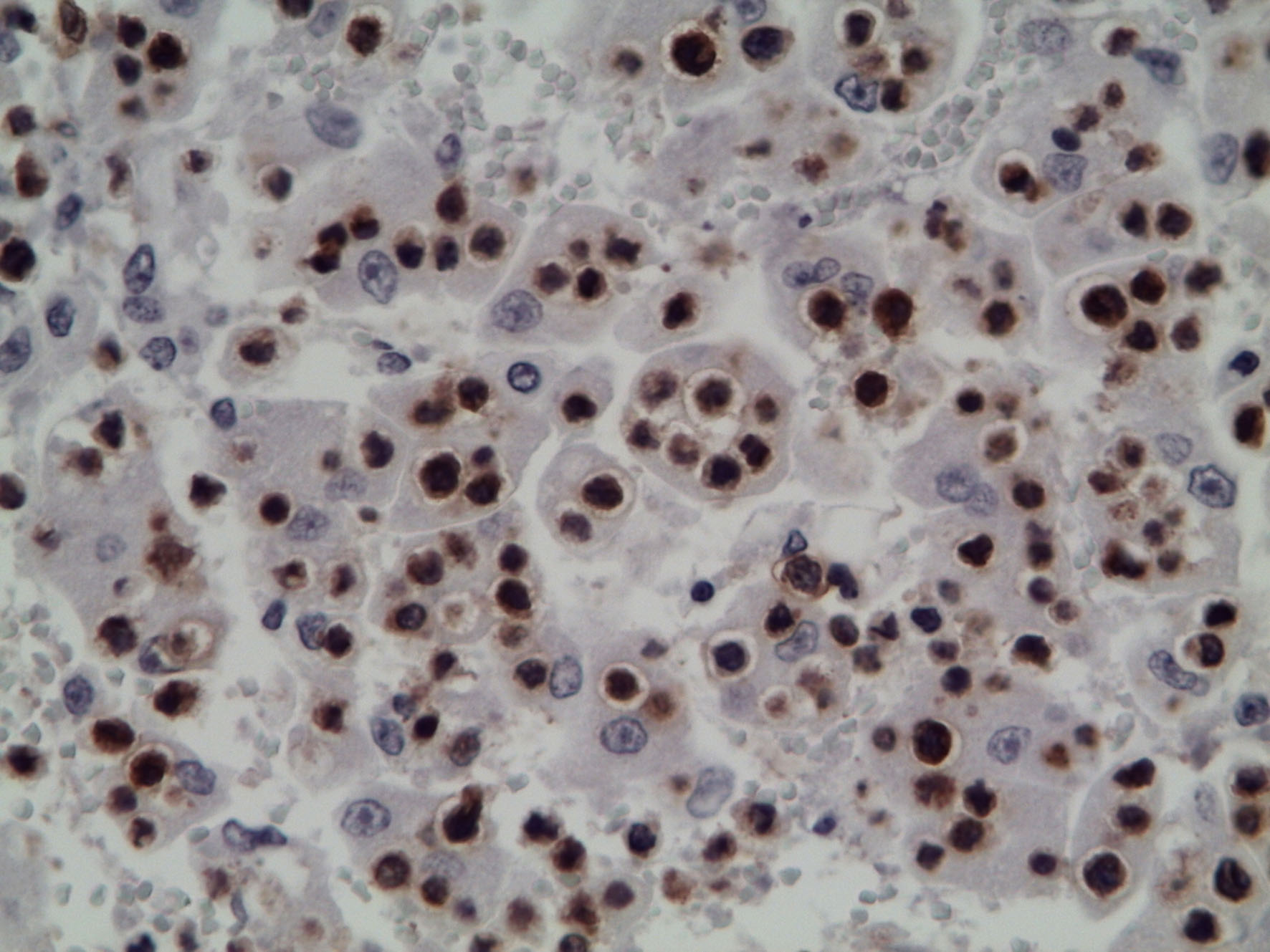
Immunohistochemical staining of mesenteric lymph node and liver showed immunoreactivity with rabbit anti-human CD3 (DakoCytomation, A 0452) and confirmed that the infiltrating cells were T lymphocytes.
Condition:
Contributor Comment:
Hepatic lesions morphologically identical to those in this cat, has been reported earlier in two cats diagnosed with lymphoblastic lymphosarcoma.3Although emperipolesis is the phenomenon of viable cells entering the cytoplasm of other cells without damage to either host cell or the engulfed cells,3 the clinical pathology of this cat, similar to the cats presented by Ossent et al,5 indicates severe hepatic failure. In addition, histology of the liver shows extensive hepatocellular dissociation.
JPC Diagnosis:
2. Liver: Regeneration, micronodular, multifocal, mild.
Conference Comment:
Given the degree of hepatocellular injury and cholestasis, conference participants unanimously preferred the term hep-atocytotropism over emperipolesis as a more representative term for the infiltrating T-cell lymphocytes in this case. This terminology was recently proposed as an alternative to emperipolesis in similar cases malignant lymphoma in the dog with distinct tropism for hepatocytes.4 Transmission electron microscopy in the dog as well as the previously reported cases in cats, showed lymphocytes within invaginations of the hepatocyte cell membrane rather than within the cytoplasm, as required for em-peripolesis.4,6,7 In addition, hepatocyto-tropism is more analogous to the epi-theliotropism present in cutaneous and intestinal T-cell lymphoma in the dog and cat.4
Several conference participants also commented on the degree of hepatic cord discohesion with dislocation of hepatic plates and diffuse loss of normal architecture in this case. Some speculated this to be partially due to moderate autolysis of the tissue. There was further discussion and some speculation that hepatic discohesion could be due to a defect in β-catenin through the Wnt signaling pathway, common in hepatic neoplasms.9 This pathway has a major role in cell adhesion to the basement membrane and cell polarity in the liver and gastrointestinal tract. In addition, β-catenin binding to E-cadherin is responsible for maintaining intercellular adhesion.5 Nor-mally, after acute hepatocellular insult, the liver is stimulated to regenerate via the Wnt pathway. The Wnt receptor signals through cell surface receptor, frizzled (FRZ), to deactivate the destruction complex, APC. The tumor suppressor APC normally binds to and destroys β-catenin.5 With the APC deactivated, β-catenin signaling drives the expression of target genes that are critical for cell cycle progression and contribute to initiation of the regeneration process.5,9 Even in the multifocal microregenerative nodules without hepatocytotropic lym-phocytes, there is hepatic cord discohesion. Regardless of the pathogenesis, the dis-association of the hepatic cords likely disrupts bile canalicular transport and sec-retion of bilirubin leading to severe hepatic cholestasis and icterus in this case.6
References:
2. Fry, M, McGavin M. Bone marrow, blood cells, and the lymphatic system. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:698-705.
3. Humble JG, Jayne WHW, Pulvertaft RJV: Biological interaction between lymphocytes and other cells. Br J Heamatol 1956;2:283-294.
4. Keller S, Vernau W, et al. Hepatosplenic and hepatocytotropic T-cell lymphoma: two distinct types of lymphoma in dogs. Vet Pathol. 2012;50:281-290. 5. Kumar V, Abbas A, Aster J. Neoplasia. In: Kumar V, Abbas A, Aster J, eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA; Saunders Elsevier; 2015: 296-297.
6. Ossent P, Stöckli RM, Pospischil A: Emperipolesis of lymphoid cells in feline hepatocytes. Vet Pathol. 1989;26:279-280.
7. Suzuki M, Kanae Y, et al. Emperipolesis-like invasion of neoplastic lymphocytes into hepatocytes in feline T-cell lymphoma. J Comp Path. 2011;144:312-316.
8. Valli V, Kiupel M, Bienzle D. Hematopoietic system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 4th ed., Vol. 3. Saunders Elsevier, Philadelphia, PA, 2016:103-104.
9. Wang E, Yeh S. et al. Depletion of β-catenin from mature hepatocytes of mice promotes expansion of hepatic progenitor cells and tumor development. PNAS. 2011;108:18384-18389.