WSC 22-23:
Conference 1
CASE I:
Signalment:
6 year-old. Female. Roya Bilbilitana. Ovine (Ovis orientalis aries)
History:
The animal presented with respiratory distress to the clinical service for ruminants (Servicio Cl?nico de Rumiantes - SCRUM) of the University of Zaragoza. The animal came from a herd of 1300 sheep and seven of them presented the same clinical signs. At clinical examination, the animal showed tachypnea and abundant foamy fluid flowing from the nostrils.
Gross Pathology:
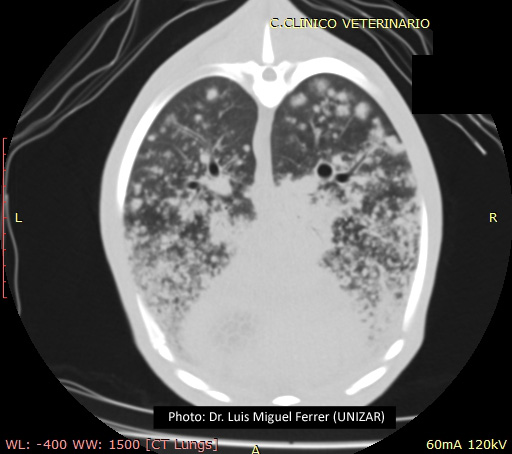
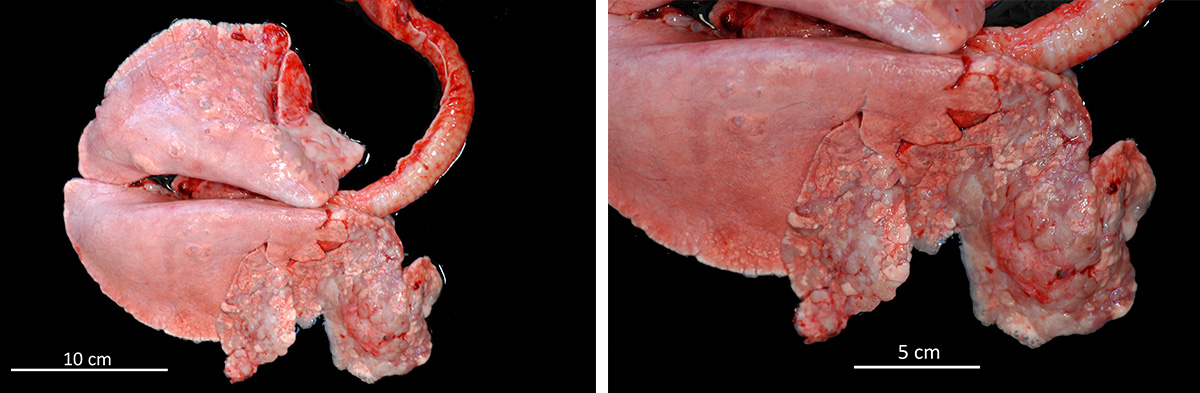
Prior to post-mortem examination, educational computed tomography (CT) scans were recorded of the thorax and evinced multiple nodules throughout the cranioventral area of the pulmonary parenchyma. At necropsy, the sheep was in poor body condition. Lungs presented a bilateral consolidation of the anterior lobes and the ventral area of the posterior ones. Lung weight was three times higher in comparison with a normal lung. There was abundant fluid in the trachea and bronchi.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
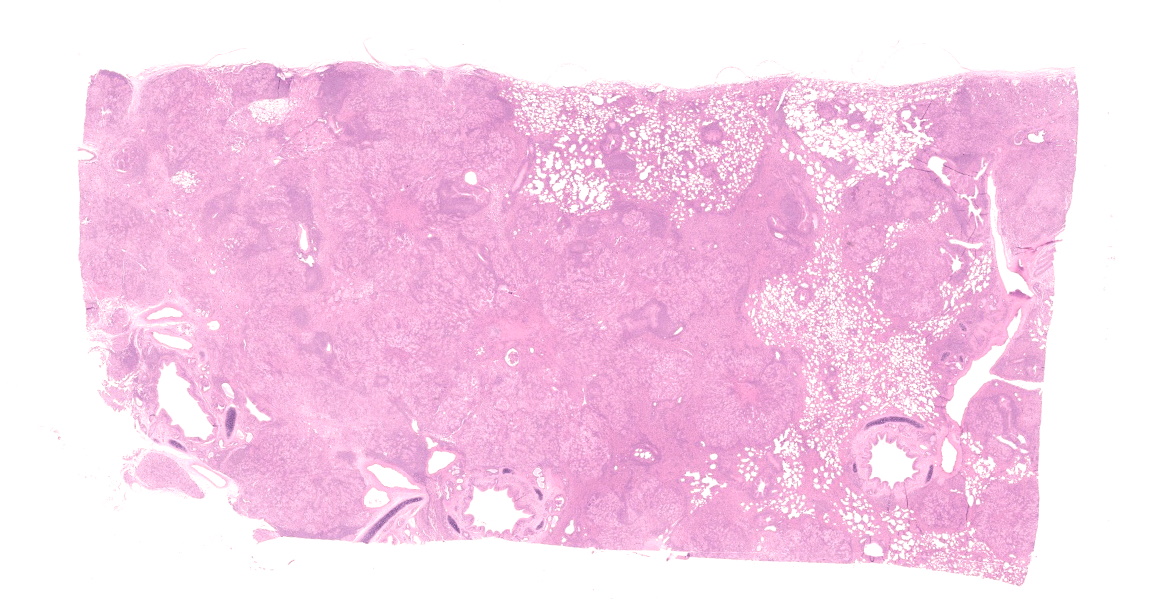
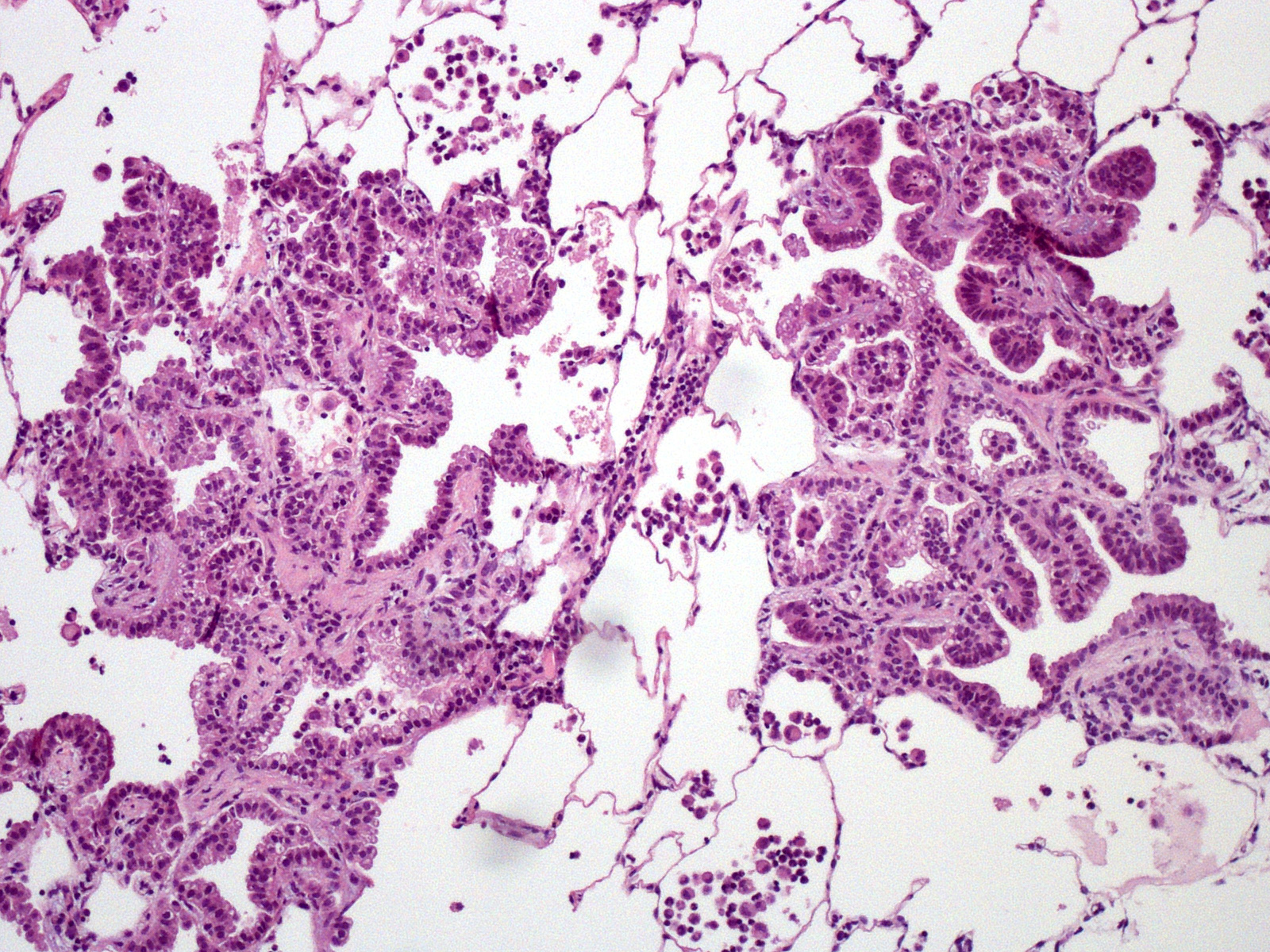
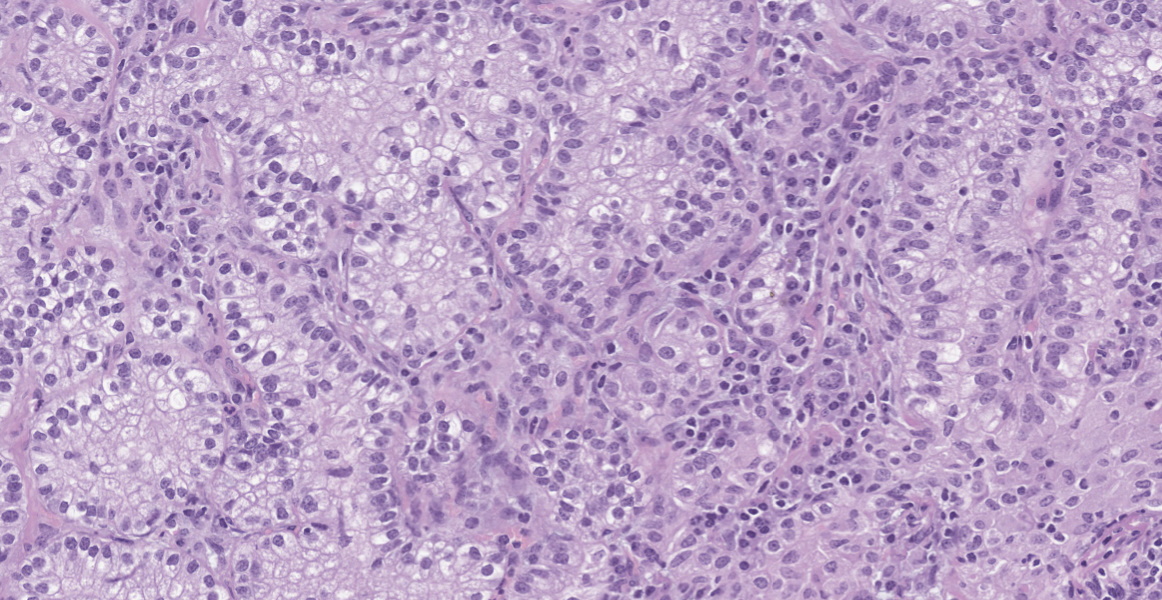
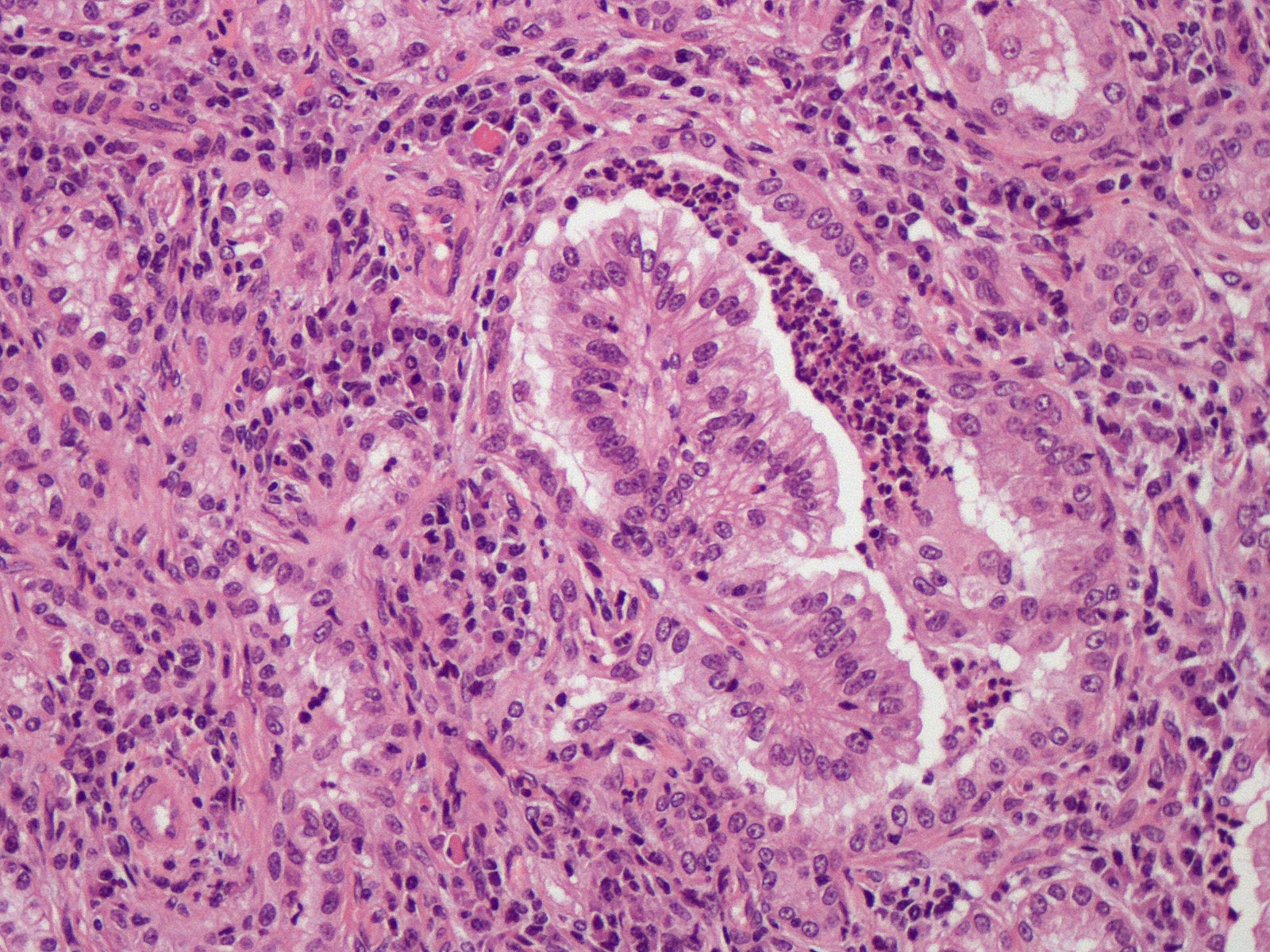
Lung. Expanding and substituting up to 70% of the pulmonary parenchyma there is a multilobulated, non-encapsulated, well-demarcated, moderately cellular and expansive neoplasm that compress adjacent airways and alveoli. Neoplastic cells form acini, tubuli and rare papillae, supported by a moderate amount of fibrovascular stroma that are multifocally expanded by dense mature collagen and fibrocytes (scirrhous reaction). Cells are polygonal to columnar, 10-15 um; show distinct cell borders, occasional apical cilia, a moderate amount of finely granular eosinophilic cytoplasm, a round to oval 7-12 um basally located nuclei with coarsely stippled chromatin and 1-2 visible nucleoli. There is moderate anisocytosis and anisokaryosis and less than one mitotic figure per 1 HPF (0.237 mm2). Multifocally, within the peritumoral non-affected parenchyma, there are intra-alveolar sheets of foamy alveolar macrophages (alveolar histiocytosis). There are intra and peritumoral aggregates of lymphocytes, plasma cells and less histiocytes as well as dense aggregates of viable and degenerated neutrophils within some tumoral acini. There are multifocal areas of alveolar edema, emphysema and prominent peribronchial lymphoid follicles (BALT hyperplasia).
Contributor's Morphologic Diagnosis:
Lung. Adenocarcinoma
Condition: Ovine Pulmonary Adenocarcinoma / Jaagsiekte / Pulmonary adenomatosis
Cause: Ovine betaretrovirus
Contributor's Comment:
This is the classical form of the Ovine Pulmonary Adenocarcinoma (OPA), also called Sheep Pulmonary Adenomatosis (SPA). This contagious pulmonary tumor is caused by an exogenous betaretrovirus, Jaagsiekte sheep retrovirus (JSRV), that target respiratory epithelial cells, mainly bronchiolar club cells and alveolar type II pneumocytes.8
There are two forms of presentation of the tumor. Classical OPA usually affects cranioventral pulmonary lobes that become solid, grey or light purple and which bronchiole are filled by abundant alveolar fluid.5 The tumor usually progress leading to respiratory embarrassment of the animal, that is evinced by dyspnea, tachypnea, exercise intolerance and, in severe cases, marked movement of the abdominal wall (abdominal lift). On the other hand, the Atypical OPA is compound of solitary or aggregated white
hard nodules. There is no production of alveolar fluid and lesion do not progress so it
usually remains subclinical and is a necropsy finding.
Microscopically, both forms are characterized by a lepidic cell pattern that can become papillary or acinar in certain areas. Classical form is usually more invasive whereas atypical form is well demarcated and surrounded by prominent infiltrate of lymphocytes and plasma cells.6,12 Metastasis to regional lymph nodes may occur in 10% of animals affected by the classical form but
dissemination to other organs is extremely rare.
Pathogenesis of the tumor is not fully understood but envelope protein has been shown to induce tumor in mice.8 The development of the classical or the atypical form seems to stem from the host immune response. Atypical forms contain more intratumoral CD4 and CD8 T-cell subsets as well as higher MHC Class II receptor expression in the tumor cells.11 It has been thought that JSRV could be lined with the induction of human lung adenocarcinoma, however new studies discard this hypothesis.6
Transformed epithelial respiratory cells are the major sites for viral replication but JSRV can also be found in lung fluid and lymphoid tissues.4 The virus is mainly transmitted by the respiratory route and close contact between animals plays a crucial role.12
Perinatal transmission via colostrum and milk is also possible.1 Most lambs become infected at very early age but only a minority (5-20%) develop OPA and they usually do between 2 and 4 years of age, evincing a long incubation period.5 Resistance to infection increases with age due to the decrease of the proliferation rate of type II pneumocytes in adults.
The main differential diagnosis of the classic form of OPA is Maedi or ovine progressive pneumonia. This disease also presents as chronic progressive respiratory problems. However, there is no fluid production and the
gross appearance is that of uncollapsed lungs and diffuse interstitial pneumonia. Gross
appearance of classical OPA could be easily confused by chronic bronchopneumonia.
Goats can also suffer from OPA but the incidence is lower.8 The transmission of the tumor between both species is possible although rare. Cattle and other animals are resistant.
Another betaretrovirus infection in sheep is Enzootic Nasal Tumor; however, both tumors are rarely seen in the same animal.
Contributing Institution:
Universidad de Zaragoza. Departamento de Patolog?a Animal
https://patologiaanimal.unizar.es/
JPCDiagnosis:
Lung: Pulmonary adenocarcinoma.
JPC Comment:
The contributor provides an excellent overview of both the classical and atypical forms of ovine pulmonary adenocarcinoma.
Jaagsiekte is an Afrikaans term meaning 'chase sickness', referring to the exacerbation of clinical signs from the stresses and physical demands of herding.9 The disease was originally described in South Africa in the 1800's and is now seen regularly in South America, Africa, Europe, Africa, and Asia.2,9 The disease has not been documented in Australia.2 In Iceland, a major outbreak in the 1930's affected up to 30% of the nation's sheep population and was eradicated by widespread culling and strict isolation measures.6
Antemortem diagnosis of OPA can be challenging. Sheep do not produce a humoral response to viral infection, so no serological tests are available. 8 PCR for pro-viral DNA in peripheral blood has low sensitivity for an individual animal (11%) but may be useful in monitoring at the flock level.6,8 Bronchoalveolar lavage fluid is more sensitive for PCR testing, however it is an impractical test to employ in the field.6 Thoracic ultrasound has also been described but has low sensitivity for lesions found deeper within the lungs and a high false positive rate. In a recent survey of sheep in the UK, only 24% of animals diagnosed with OPA via ultrasound were positive on histologic and IHC examination.3
As the contributor stated, the gross appearance of OPA can mimic that of chronic bronchopneumonia, and in one study of OPA in abattoirs in Ireland, there was a 89% false-positive rate for diagnosis of OPA based on gross lesions alone.6 Histologic examination improves diagnostic rates, however confirmatory IHC or RT-PCR may be needed to rule out the less common non-JSRV associated pulmonary adenocarcinoma or to diagnose OPA masked by concurrent disease (i.e. bronchopneumonia).6
Table 1-1, adapted from Fenner's Veterinary Virology, summarizes the major retroviruses that induce neoplasia in animals. Both the enzootic nasal tumor retroviruses and JSRV are classified as a trans-activating retroviruses as neoplastic transformation stems from expression of the viral env gene, compared to cis-acting retroviruses that alter expression of the host proto-oncogenes. 10
During the conference, the moderator, Dr. Corrie Brown from the University of Georgia, described clinical and pathologic features of the cases she has seen during her work overseas, including the wheelbarrow test. Lifting an affected animal's hindlimbs like a wheelbarrow will cause frothy fluid pour from the nostrils; this is secondary to agonal exasperation of severe pulmonary edema. Dr. Brown also reminded conference participants of the impact this diagnosis can have on small farmers in low-income countries: if one animal is affected, the farmer will invariably lose more of their herd - and a significant portion of their livelihood.
|
Table 1-1: Retroviruses that Induce Tumors in Animals, Adapted from Fenner's Veterinary Virology.10 |
||
|
Genus |
Species |
Syndrome |
|
Alpharetrovirus |
Avian leukosis virus |
Leukosis (lymphoma, leukemia), nephroblastoma in fowl |
|
Rous sarcoma virus |
Sarcoma in fowl |
|
|
Avian myeloblastosis virus |
Myeloblastosis in fowl |
|
|
Betaretrovirus |
Mouse mammary tumor virus |
Mammary carcinoma in fowl |
|
Mason-Pfizer monkey virus |
Sarcoma and immunodeficiency disease in monkeys |
|
|
Ovine pulmonary adenocarcinoma virus (Jaagsiekte virus) |
Pulmonary adenocarcinoma in sheep |
|
|
Enzootic nasal tumor virus |
Enzootic nasal adenocarcinoma in sheep and goats |
|
|
Gammaretrovirus |
Feline leukemia virus |
Leukemia in cats |
|
Feline sarcoma virus |
Sarcoma in cats |
|
|
Murine leukemia and sarcoma viruses |
Leukemia, lymphoma, and sarcoma in mice |
|
|
Deltaretrovirus |
Avian reticuloendotheliosis virus |
Reticuloendotheliosis in fowl |
|
Bovine leukemia virus |
Leukemia (B cell lymphoma) in cattle |
|
References:
- Borobia M, De Las Heras M, Ramos JJ, et al. Jaagsiekte Sheep Retrovirus Can Reach Peyer's Patches and Mesenteric Lymph Nodes of Lambs Nursed by Infected Mothers. Vet Pathol; 2016; 53(6):1172-1179.
- Caswell JL, Williams KJ. The respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 2. Philadelphia, PA: Elsevier Saunders; 2016: 559-562.
- Davies P, Strugnell B, Waine K, et al. To scan or not to scan? Efficacy of transthoracic ultrasonography for ovine pulmonary adenocarcinoma screening in a large commercial UK sheep flock. Vet Rec. 2022; e1578.
- De Las Heras M, De Martino A, Borobia M, et al. Solitary Tumours Associated with Jaagsiekte Retrovirus in Sheep are Heterogeneous and Contain Cells Expressing Markers Identifying Progenitor Cells in Lung Repair. J Comp Pathol; 2014; 150:138-147.
- Griffiths DJ, Martineau HM, Cousens C. Pathology and Pathogenesis of Ovine Pulmonary Adenocarcinoma. J Comp Pathol. 2014; 142:260-283.
- Lee AM, Wolfe A, Casside JP, et al. First confirmation by PCR of Jaagsiekte sheep retrovirus in Ireland and prevalence of ovine pulmonary adenocarcinoma in adult sheep at slaughter. Ir Vet J. 2017;70(33): 1-12.
- Miller AD, De Las Heras M, Yu J, et al. Evidence against a role for jaagsiekte sheep retrovirus in human lung cancer. Retrovirology. 2017; 14:3
- Monot M, Archer F, Gomes M, Mornex J-F, Leroux C. Advances in the study of transmissible respiratory tumours in small ruminants. Vet Microbiol. 2015; 181:170-177.
- Murphy B. Chapter 14: Retroviruses. In: MacLachlan NJ, Dubovi EJ eds. Fenner's Veterinary Virology. 5th ed. Boston, MA: Academic Press, 2017: 269-298.
- Niewiesk S, Oglesbee M. Chapter 3 - Pathogenesis of Viral Infections and Diseases. In: MacLachlan NJ, Dubovi EJ eds. Fenner's Veterinary Virology. 5th ed. Boston, MA: Academic Press, 2017: 47 - 78.
- Sharp JM, De Las Heras M. Contagious respiratory tumours. In: Aitken ID, editor. Diseases of Sheep. Fourth Edition. Oxford (UK): Blackwell Publishing; 2007. 211-217.
- Summers C, Benito A, Ortin A, et al. The distribution of immune cells in the lungs of classical and atypical ovine pulmonary adenocarcinoma. Vet Immunol Immunopathol. 2012; 146:1-7.