Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 9
November 1st, 2017
CASE I: 17-38-W (JPC 4101294).
Signalment: 26-week-old, male, FVB/N, Mus musculus, mouse.
History: This mouse harbored 3 transgenes: Twist1-tetO7-luc, CCSP-rtTA, and tetO-KrasG12D. The mouse had been administered doxycycline in the drinking water since 5 weeks of age to induce transgenes and promote lung tumorigenesis. The doxycycline-treated water was replaced weekly during this time. This mouse was unexpectedly found dead just prior to the intended sacrifice date.
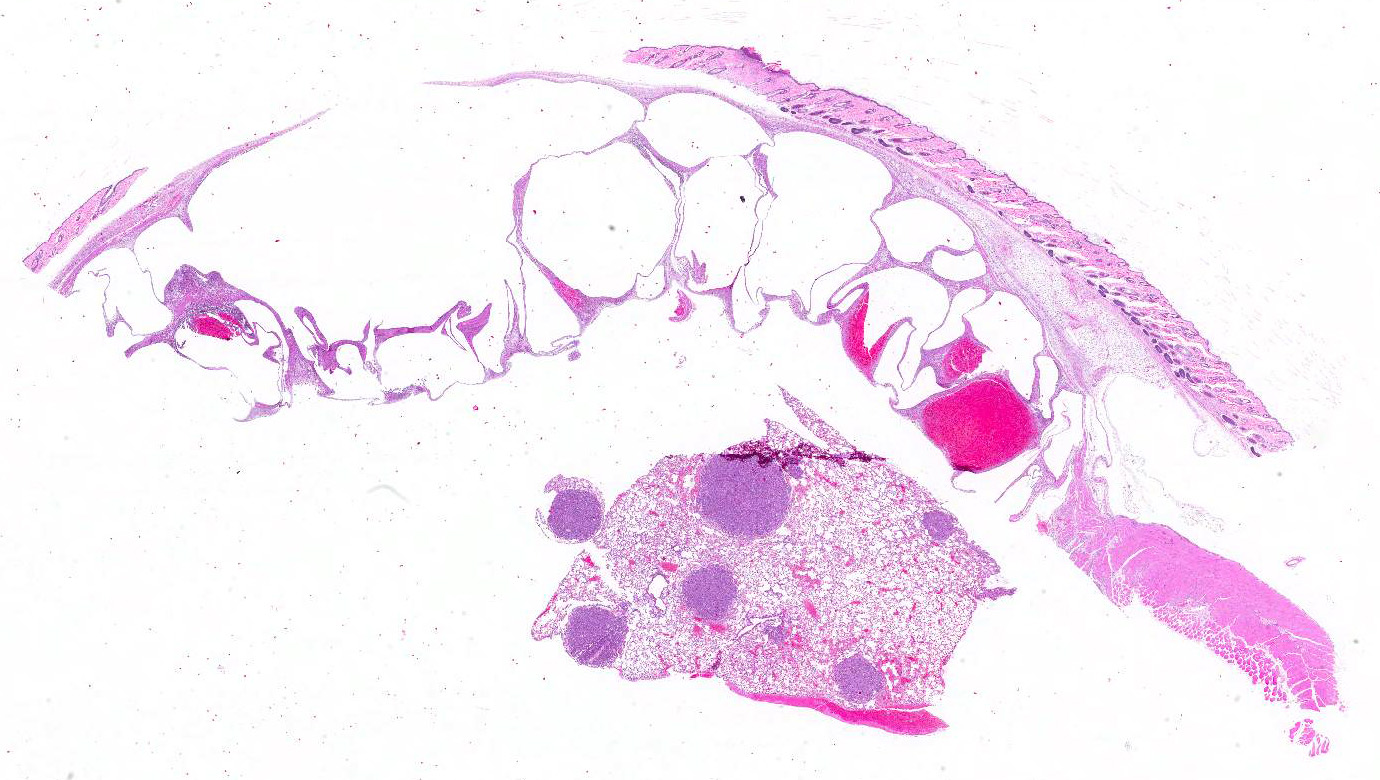
Gross Pathology: The lungs contained numerous white-tan, firm, lung tumors that ranged from pinpoint to 3 x 3mm diameter in all lobes. Additionally, there was a 3 x 4x 0.75cm multilobulated, mass expanding the left abdominal body wall adjacent to the diaphragm (Fig 1). On cut section, the mass was composed of numerous variably sized air and blood-filled cysts. A crackling sound could be elicited upon compression of this body wall mass (subcutaneous crepitation), even post-fixation.
Laboratory results: None performed.
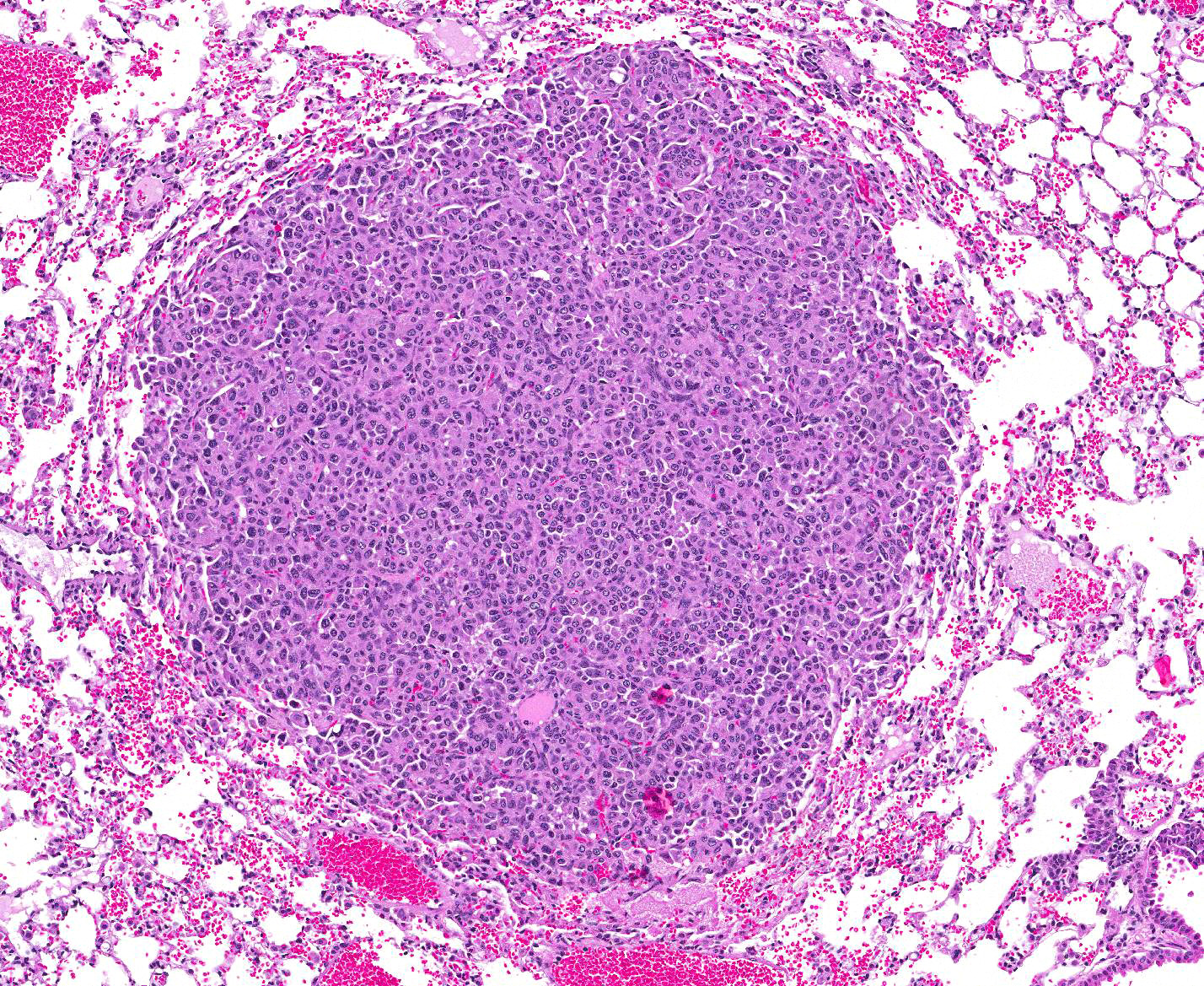
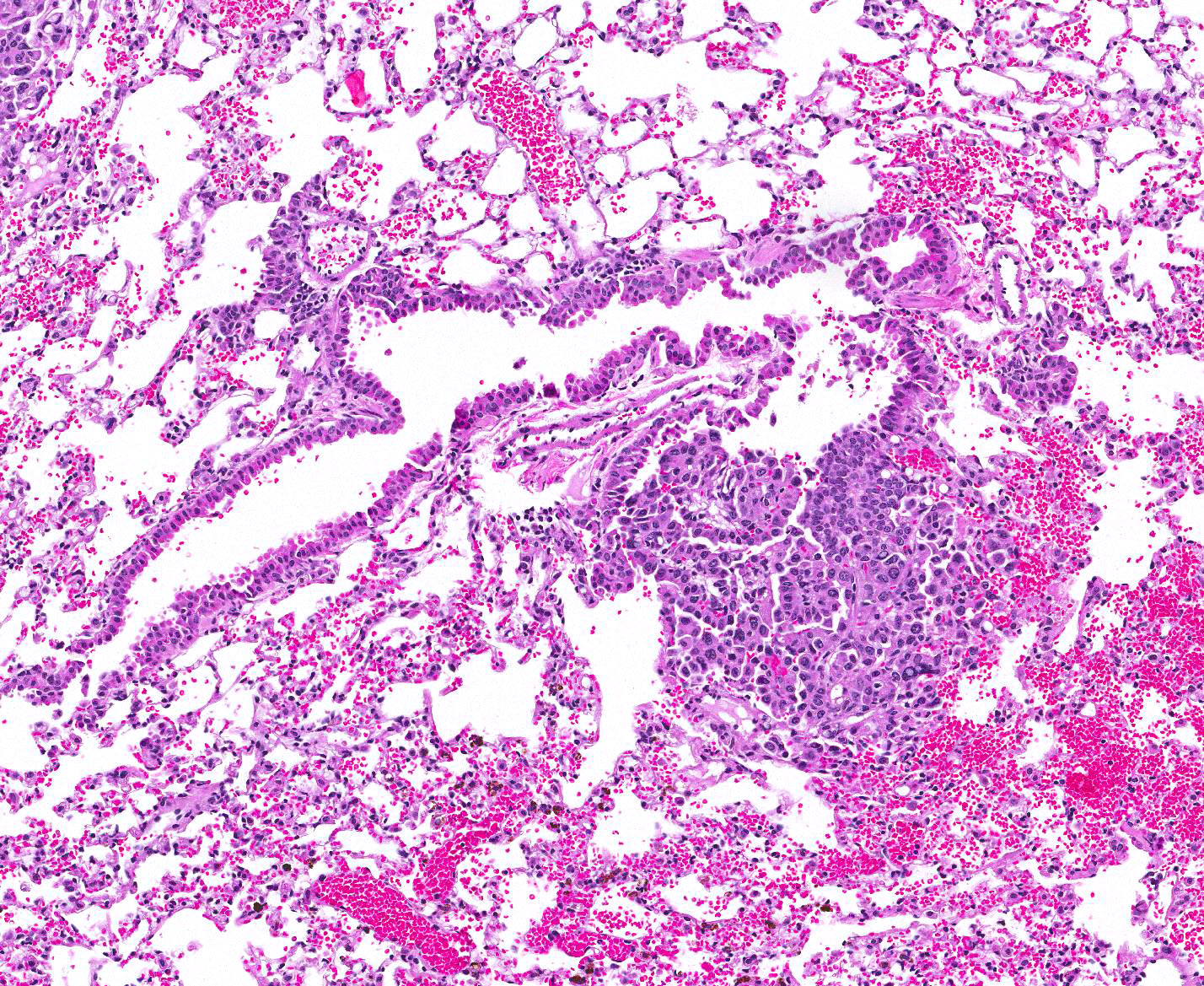
Microscopic Description: Lung: Compressing adjacent pulmonary parenchyma are mulfocal, variably-sized, up to 3mm diameter, unencapsulated, well-demarcated, expansile, neoplastic foci composed of one or more layers of cuboidal to columnar epithelial cells arranged in disorganized glands and packets, supported by a fine fibrovascular stroma. Neoplastic cells have variably-distinct cell borders, scant to moderate amounts of fibrillar eosinophilic to amphophilic cytoplasm, and round to oval nuclei moderately pleomorphic nuclei with finely-stippled chromatin, and 0?4 variably distinct nucleoli. The mitotic rate is less than 1 per 10 HPF. Occasionally, neoplastic cells with prominent nuclear invaginations are present. Within some neoplastic foci, there are areas of necrosis characterized by sloughed and/or pyknotic neoplastic cells, degenerative neutrophils, and cell debris. Necrotic foci are most common in the central portions of larger neoplasms. Smaller neoplastic foci can be seen extending from alveolar or bronchiolar epithelium. Within the adjacent parenchyma, alveoli often contain red blood cells and fibrin. In some sections, there are numerous alveolar macrophages containing red blood cells and/or hemosiderin.
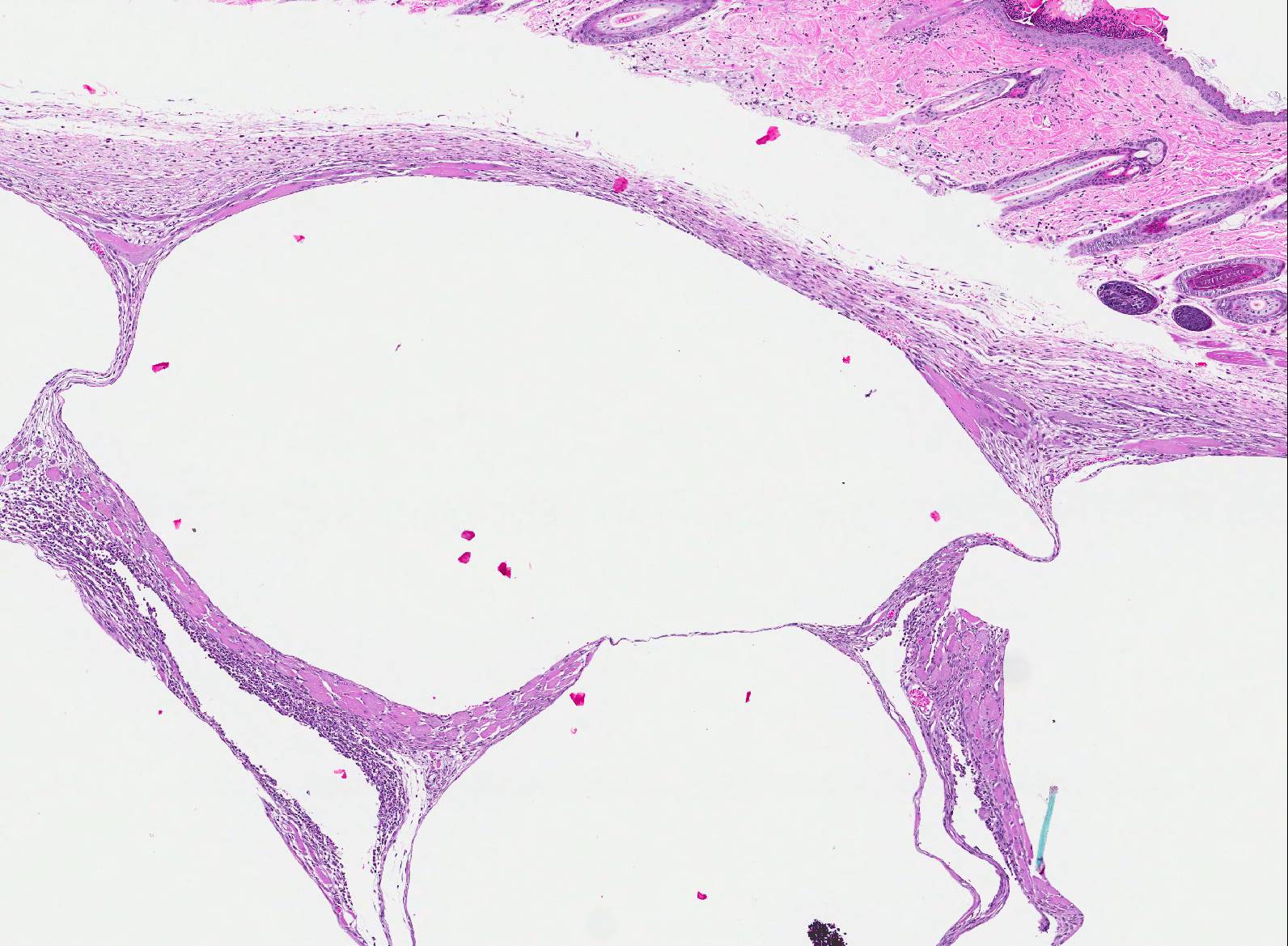
Haired skin, body wall: Diffusely expanding the subcutis and underlying skeletal muscle, are numerous round to irregular cysts (up to 3mm in diameter) filled with clear space (air) and occasionally red blood cells, and/or inflammation and necrosis characterized by neutrophils and macrophages with fibrin and cell debris. Cystic spaces are separated by elongated collagen bundles, pre-existing skeletal muscle bundles, foci of fibroblasts, hemorrhage, and the previously described inflammation and necrosis. The skeletal muscle in this region is multifocally characterized by sarcoplasm that is pale and swollen with internalized nuclei (degeneration), or brightly eosinophilic with loss of cross striations (necrosis).
Contributors Morphologic Diagnosis:
1. Lung: Adenocarcinoma, multifocal.
2. Haired skin and body wall: Emphysema, subcutaneous and intermuscular, with hemorrhage, and subacute myositis.
Contributors Comment: Most human lung cancers are adenocarcinomas carrying somatic mutations in the genes that encode the EGFR/KRAS/BRAF pathway.4 Mouse models utilizing the expression of these transgenes are commonly utilized as models for the human disease. Rat Clara cell secretory protein (Ccsp)-rtta activator mice were described in 2000, and provide models in which expression is conditionally controlled in respiratory epithelial cells in the lung, altering lung morphogenesis, differentiation, and proliferation.10 Additionally, it has been shown that Twist1 plays an important role in both the acceleration and maintenance of KrasG12D -induced autochthonous lung tumorigenesis.11 As can be expected based on the individual role of each of these transgenes, induction of Twist1-tetO7-luc, CCSP-rtTA, and tetO-KrasG12D in the mouse produces model that may be used to study tumorigenesis in human adenocarcinoma and develop potential treatments.
Subcutaneous edema is often seen in the neck, mediastinum, and retroperitoneal soft tissues secondary to trauma.7 Additionally, it can be generated by gas forming bacteria secondary to infection or it may arise spontaneously when the pressure gradient between the air-filled alveoli and their surrounding interstitial space is sufficient to cause alveolar rupture.7
In this case, the emphysema developed as a bronchocutaneous fistula that presumably occurred after the rupture of one of the many adjacent lung tumors. Bronchocutaneous fistulas can occur in human lung cancer patients. They are a very rare complication, and are the extended version of a bronchopleural fistula, a direct communication between the pleura and bronchial system or the lung parenchyma.5 Most bronchopleural fistulae are postoperative complications of surgical resections of lung, secondary to chemotherapy, radiation treatment, chronic inflammation or infection or a result of internal or external chest trauma.5,8 Although rare, there are reports of bronchocutaneous fistulas developing in patients that have not received surgical resections, chemotherapy or radiotherapy.6
The development of numerous pulmonary adenocarcinomas was expected in the mouse, but the large area of subcutaneous emphysema in the abdominal body wall was not. This has never been described as a potential complication in an inducible lung tumor mouse models. Perhaps, with the continued use of transgenic lung tumor mouse models, rare complications like these may be seen more often by the evaluating comparative pathologists.
JPC Diagnosis: 1. Lung: Pulmonary adenomas, multiple, FVB/N (Mus musculus), mouse.
2. Haired skin and subcutis: Emphysema, diffuse, subacute, severe.
Conference Comment: In mice, primary pulmonary adenomas and adenocarcinomas are the most frequent tumor. In highly susceptible strains, such as A strain mice, tumors can become apparent by 3-4 months old with 100% prevalence by 18-24 months. The high susceptibility of this strain is due to activation of K-ras in the tumors that affect their K-ras allele. In less susceptible strains such as: Outbred Swiss, FVB, BALB/c, 129, and B6:129 hybrids, a predisposition for tumor growth comes from infection with various viral infections (such as Sendai virus) or chemical carcinogens.1 Primary pulmonary tumors are considered benign until they reach 3mm in diameter, after which they are considered adenocarcinomas or carcinomas. In this case, regardless of the mouse strain, we relied on the INHAND (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) method and determined the size of the tumors to be less than 3mm and, therefore, categorized them as adenomas.9
In domestic animal species, primary pulmonary neoplasms are most common in dogs and cats and epithelial tumors are the most common type. Of note, intrapulmonary metastasis is common, and therefore the presence of neoplastic cells within vessels of a pulmonary tumor is not necessarily helpful in distinguishing a primary from a secondary neoplasm. The histologic characteristics that support a primary neoplasm are as follows: Absence of a primary tumor in another organ, presence of single large mass with or without smaller metastases, detection of thyroid transcription factor-1 (TTF-1), mucous production or ciliated cells (both of which are rare in primary tumors).3 Recently, a study comparing the efficacy of immunohistochemistry of surfactant protein A (SP-A), napsin A, and TTF-1 in diagnosing canine pulmonary carcinomas found that SP-A and napsin A are both useful markers, but that SP-A is the most sensitive and specific and should be paired with napsin-A or TTF-1 to improve detection and differentiation of pulmonary carcinomas from tumors metastatic to the lung.2 The most frequent lung tumors in dogs are minimally invasive, lepidic predominant, and papillary predominant adenocarcinomas. The grading system in dogs is based on the following criteria: differentiation, degree of nuclear pleomorphism, mitotic rate, nucleolar size, tumor necrosis, fibrosis, and demarcation of the mass. The parameters most predictive of outcome are degree of differentiation, mitotic rate (cutpoints of >1 and >3 per high-power field), necrosis (> 50% of the tumor), and nucleolar size. Staging provides prognostic information, at least in dogs, and is based on number of tumors present (>1=T2), invasion of adjacent tissues (T3), or neoplastic cells present in lymph nodes or other organs (metastasis), both of which indicate a poor prognosis. With pulmonary tumors, invasion refers to neoplastic cells extending into tumor stroma, blood or lymphatic vessels, or pleura, but not extension into adjacent lung tissue. In older cats, pulmonary adenocarcinoma is quite common with a unique pattern of metastasizing to the digits, specifically the dermis on the dorsum of the distal phalanx and beneath the footpad.3
Division of Laboratory Animal Resources
University of Pittsburgh
References:
1. Barthold SW, Griffey SM, Percy DH. Pathology of Laboratory Rodents and Rabbits. Ames, IA: John Wiley & Sons, Inc.; 2016:112-113.
2. Beck J, Miller MA, Frank C, DuSold D, et al. Surfactant protein a and napsin a in the immunohistochemical characterization of canine pulmonary carcinomas: comparison with thyroid transcription factor-1. Veterinary Pathology. 2017;54(5):767-774.
3. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol. 2. 6th ed. St. Louis, MO: Elsevier; 2016:495-497.
4. Ding L, Getz G, Wheeler DA, Mardis ER, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008; 455: 10691075.
5. Fraser A, Nolan RL. Malignant bronchosubcutaneous fistula presenting as subcutaneous emphysema. J Thorac Imag. 2002; 17: 319321.
6. Kumar A, Foden AP. Spontaneous subcutaneous emphysema secondary to a malignant bronchocutaneous fistula in a patient who had not received chemotherapy or radiotherapy. Am J Respir Crit Care Med. 2012; 185: A4391.
7. Maunder RJ, Pierson DJ, Hudson LD. Subcutaneous and mediastinal emphysema. Pathophysiology, diagnosis, and management. Arch Intern Med. 1984; 144(7): 1447-1453.
8. Powner DJ, Bierman MI. Thoracic and extrathoracic bronchial fistulas. Chest. 1991; 100: 480486.
9. Renne R, Brix A, Harkema J, Herbert R, et al. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicologic Pathology. 2009;37(7 Suppl):5S-73S.
10. Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem. 2000; 275(16): 11858-11864.
11. Tran PT, Shroff EH, Burns TF, Thiyagarajan S, et al. Twist1 suppresses senescence programs and thereby accelerates and maintains mutant Kras-induced lung tumorigenesis. PLoS Genet. 2012;8(5):e1002650.doi: 10.1371/journal.pgen.1002650.