WSC 2023-2024, Conference 20, Case 1
Signalment:
12-year-old, female spayed Schipperke dog (Canis familiaris)
History:
A 12-year-old, female spayed Schipperke dog presented with multisystemic disease including severe enteropathy, hepatopathy, pneumonia, and dermatitis. The patient had been on long-term budesonide, prednisone, and dexamethasone for inflammatory bowel disease.
Gross Pathology:
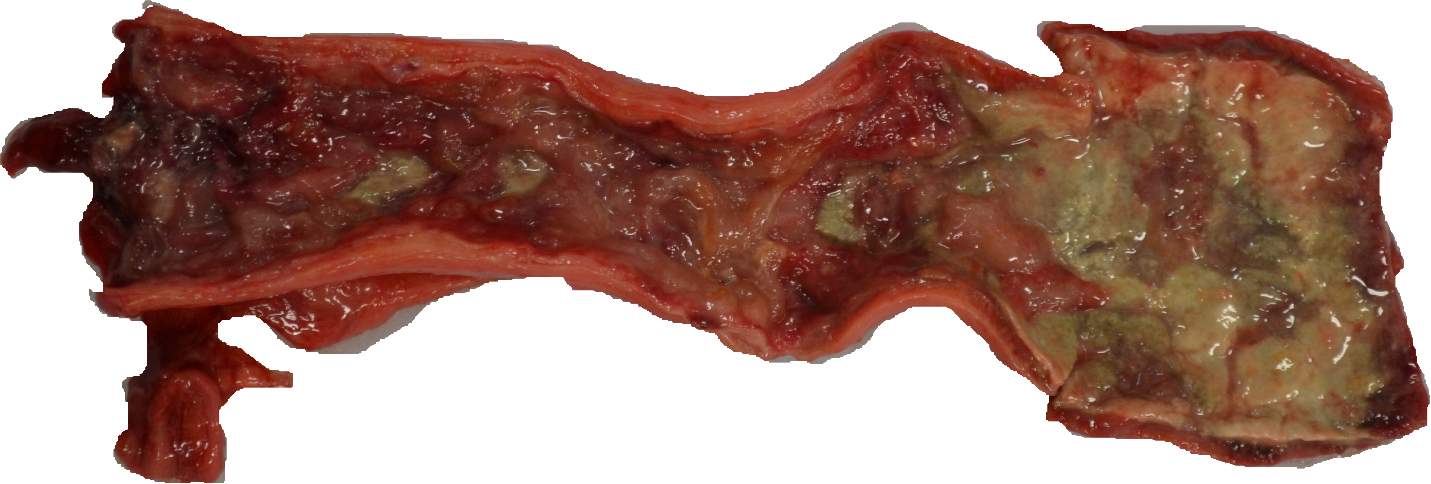
Gross examination revealed severe ulceration of the mucosal surface of the colon, with abundant necrotic and fibrinous debris overlying the sites of mucosal injury. The medial/internal aspect of the right ear pinna was crusted with regionally extensive ulceration and mild hemorrhage. The skin, sclera, gingiva, and subcutaneous fat were mildly icteric. The liver was friable and homogenously pale tan and moderately enlarged, with slightly rounded margins. All liver lobes had disseminated pale tan foci ranging from 3-5 mm in diameter. The spleen was mildly enlarged with a meaty consistency. The lungs were diffusely mottled pink to purple, with similar disseminated pale tan foci as the liver. The bone marrow (left femur) was diffusely reddened.
Laboratory Results:
Neospora caninum PCR: DNA detected in colon samples.
Toxoplasma gondii PCR: DNA not detected in colon.
Leishmania spp. PCR: DNA not detected in liver samples.
Microscopic Description:
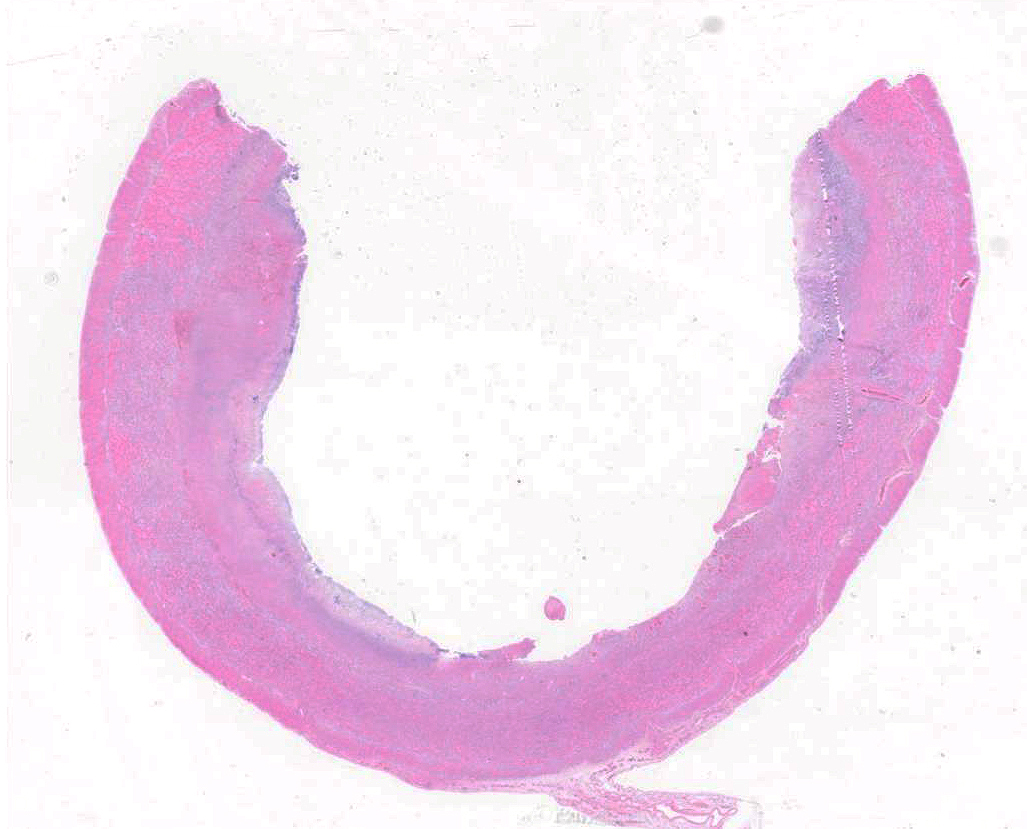
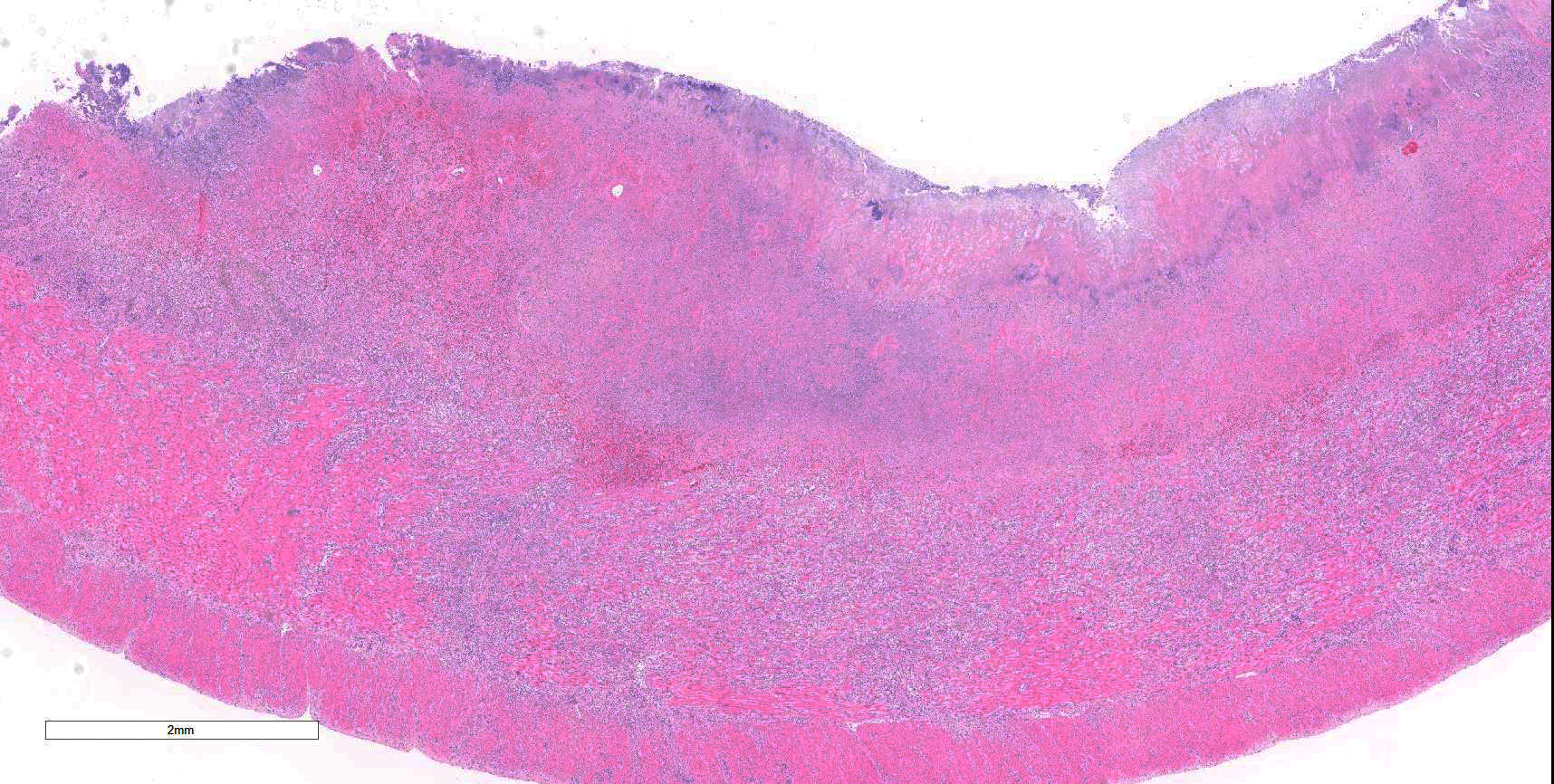
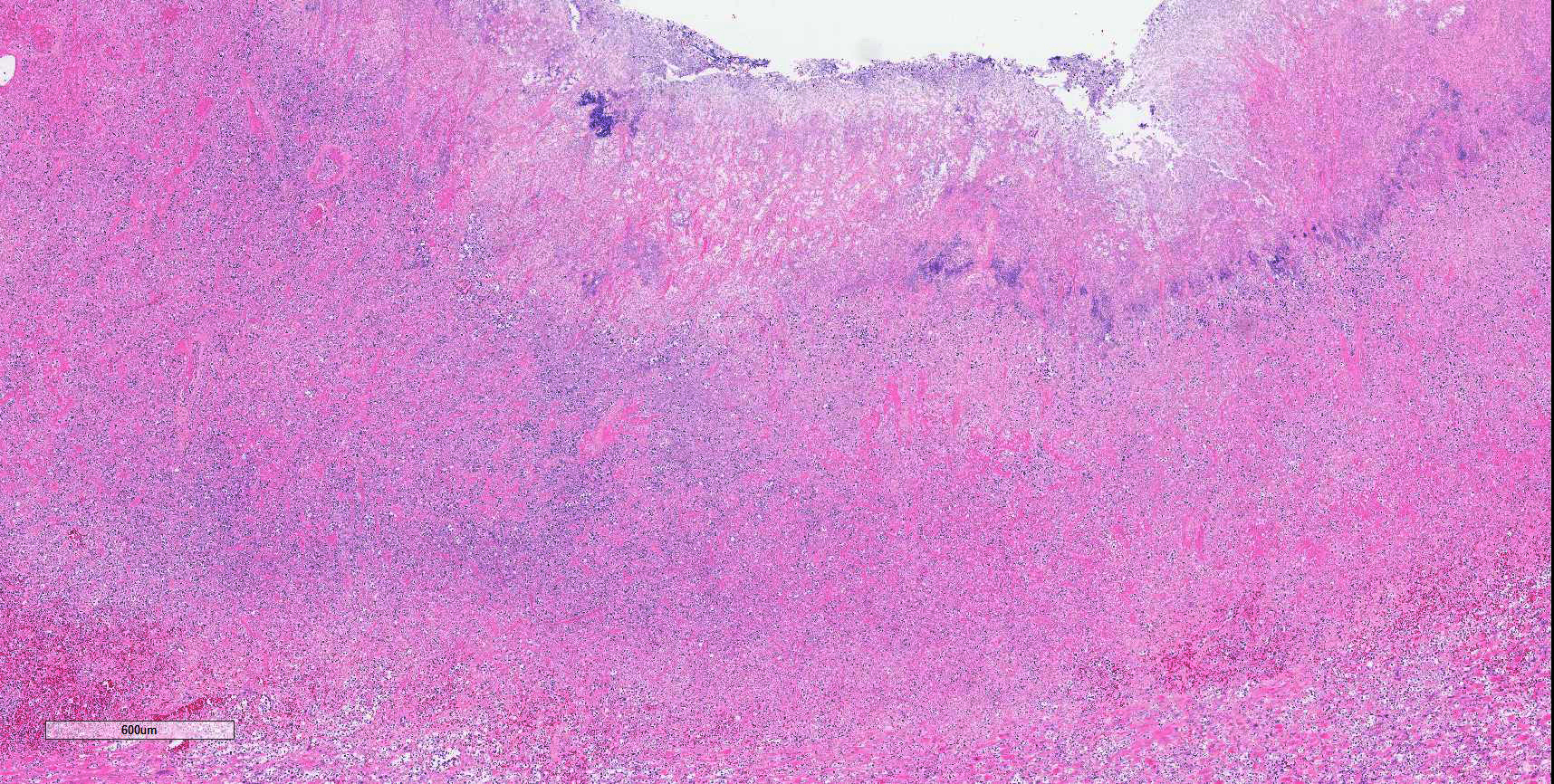
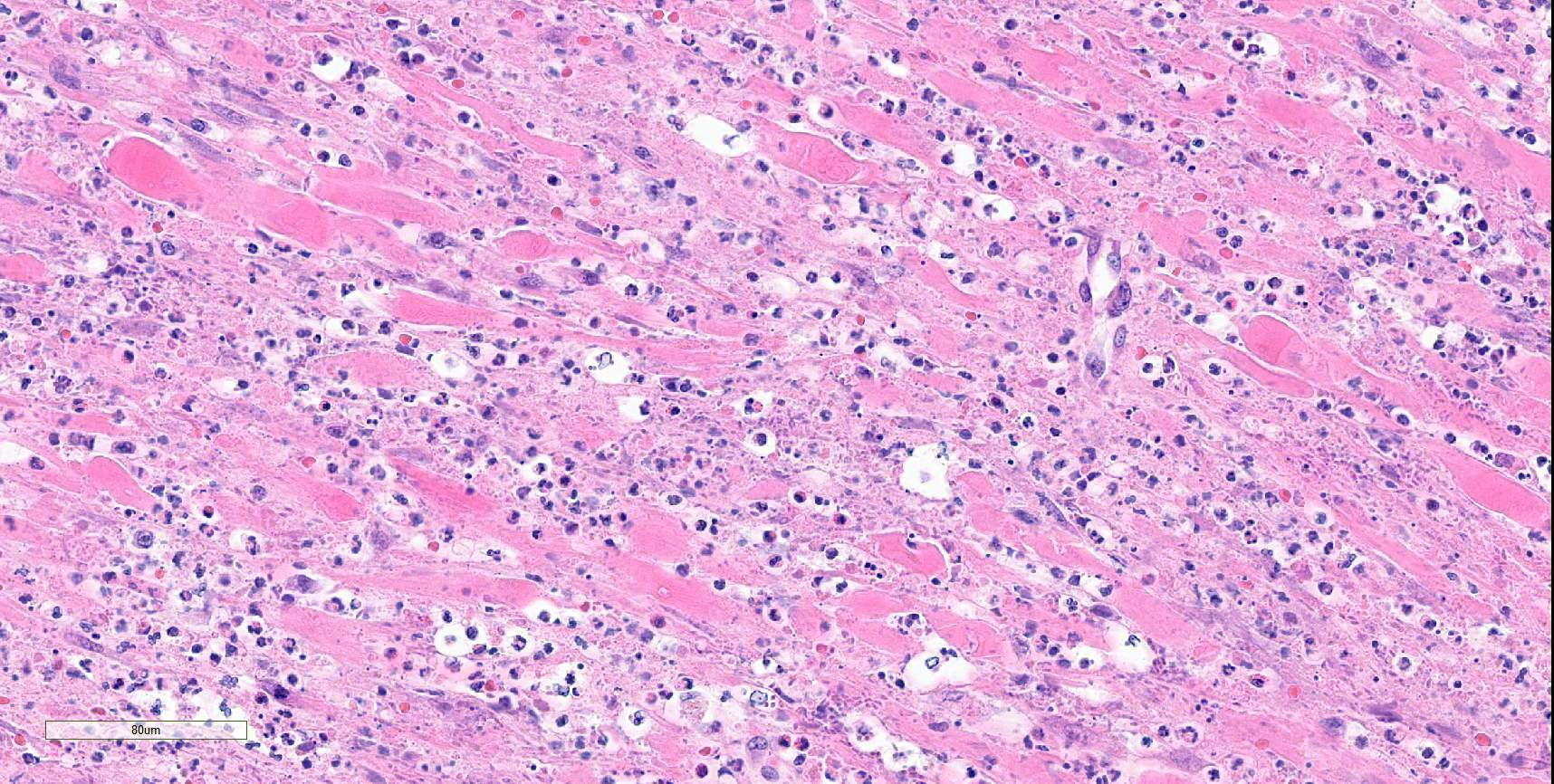
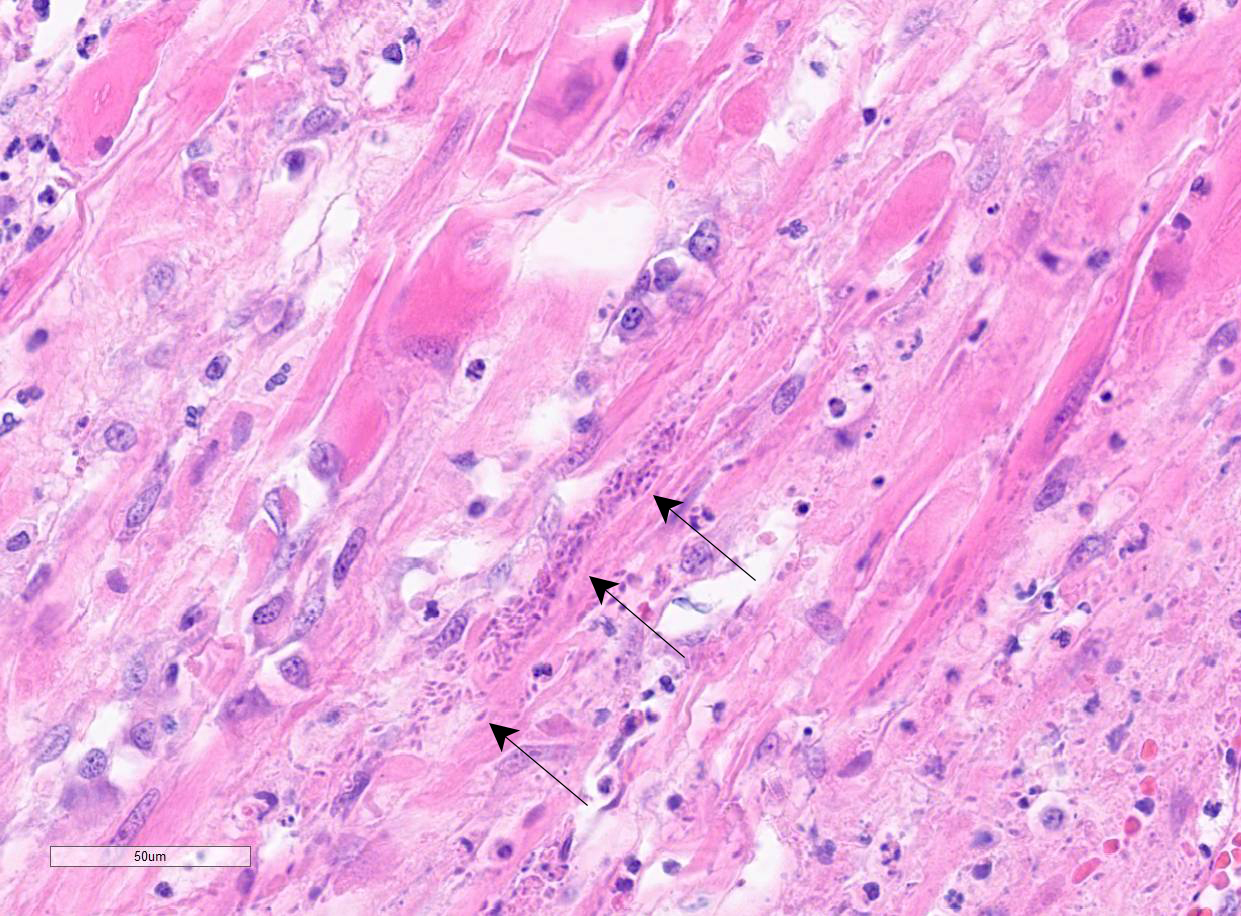
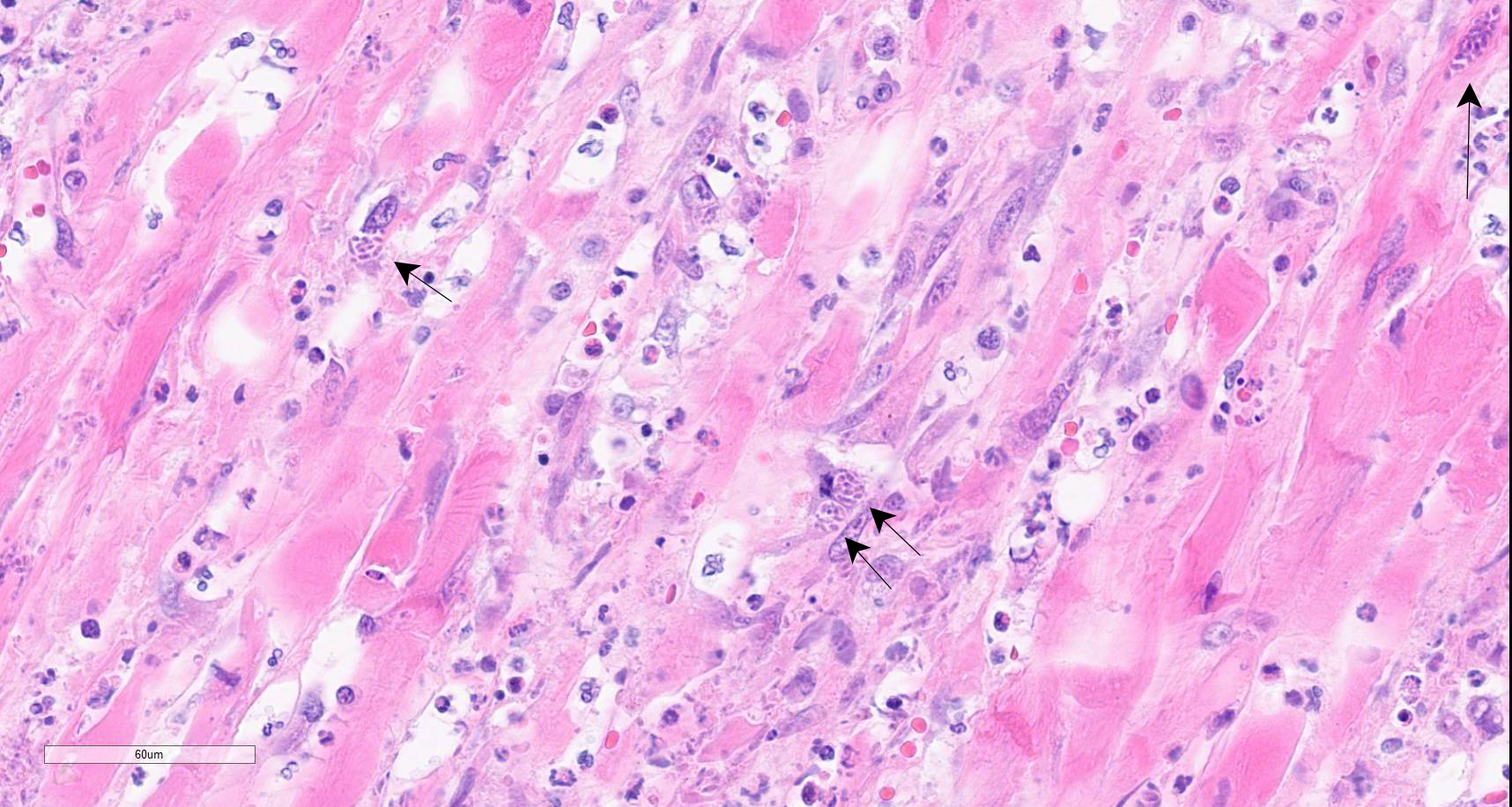
Colon: There is marked multifocal to coalescing ulceration of the colonic mucosa. Overlying the injury are thick mats of necrotic cellular debris, fibrin, large numbers of histiocytes and degenerate neutrophils, entrapped mixed bacteria, and low amounts of hemorrhage. Inflammation, necrosis, and edema extend deep throughout the submucosa and muscularis, disrupting the mural architecture. Throughout areas of inflammation and within muscle fibers of the muscularis are myriads of unicellular tachyzoite parasitic organisms. Tachyzoites are 2-3 µm long by 1-2 µm wide, almond-shaped, with a round basophilic nucleus, and perinuclear clearing extending to both tapered ends. Rarely there are encapsulated clustered of tachyzoites (cysts). These organisms are also present within epithelial cells and endothelial cells.
Protozoal tachyzoites and cysts stain positive for Giemsa and negative for GMS and Gram stain.
Contributor’s Morphologic Diagnosis:
Colon: Severe, multifocal to coalescing, necroulcerative colitis with myriad intralesional protozoa.
Contributor’s Comment:
In addition to the lesions in the colon, tachyzoites were identified in the liver, spleen, and skin, and fewer numbers were identified in the bone marrow and lungs. There was variable necrotizing to histiocytic inflammation in each affected tissue. The morphology of the tachyzoites, along with the detection of Neospora caninum by PCR, are diagnostic of an unusual case of disseminated neosporosis. Differentials considered included Toxoplasma gondii and Leishmania spp. Toxoplasmosis cannot be distinguished from neosporosis histologically. Molecular diagnostics such as PCR have been instrumental in the diagnosis and differentiation of the two diseases. Leishmaniasis typically can be distinguished from the apicomplexan parasites due to the presence of the kinetoplast within amastigotes, but this is not always readily identifiable in standard H&E preparations.
Neospora caninum is an apicomplexan parasitic organism with a heteroxenous life cycle, for which canids are the definitive host.4,7,8 In canids, disease is uncommon but typically presents as neuromuscular disease.8,12,13 In puppies (<6 months) the typical clinical presentation is an ascending, progressive paralysis with rigidity and muscle atrophy, mostly of the pelvic limbs.3,4,5,12 Adult dogs may experience recrudescence of latent infections, with multifocal CNS signs and polymyositis. The gross findings may include embolic foci of necrosis and hemorrhage in the brain and spinal cord and white streaking, corresponding to necrosis, in the muscles. Tachyzoites can be found free in the tissues or within histiocytes, endothelial cells, neurons, and epithelial cells, and are usually accompanied by marked necrosis and granulomatous inflammation.1,2,5,6,12
Cases of myocarditis, pneumonia, dermatitis, and dissemination are less common and have typically been associated with immunosuppression including steroid or chemotherapeutic administration.4,9,10,11 In this case, the patient was on immunosuppressive doses of budesonide, prednisone, and dexamethasone for presumptive inflammatory bowel disease. Additionally, the patient had a history of copraphagia as well as regular exposure to cattle. Cattle are the most common intermediate host, for which mid-term abortion (at about 5-6 months gestation) is the most common clinical presentation.3,4,5,7,8, The absence of gross lesions is common in cattle; however, if present, the pathognomonic lesion in aborted fetuses is multifocal foci of necrosis in brain tissue.5,6,14
Contributing Institution:
Veterinary Diagnostic Laboratories|
Colorado State University
Fort Collins, CO
http://csu-cvmbs.colostate.edu/vdl/Pages/default.aspx
JPC Diagnosis:
Colon: Colitis, necrotizing, multifocal to coalescing, transmural, with vasculitis, thrombosis, and numerous intramyocytic, intraendothelial, and free zoites.
JPC Comment:
Neospora caninum shares many histologic and biologic features with the closely related apicomplexan parasite Toxoplasma gondii; however, there are key differences between the two related to host range, virulence factors, and pathogenesis.4 N. caninum follows a life cycle in which canids, including domestic and wild dogs, coyotes, wolves, and dingoes, serve as definitive hosts where sexual replication occurs, and a variety of intermediate hosts provide the ecological niche for asexual replication. As the contributor notes, the most common intermediate host is cattle, and it appears that the N. caninum life cycle is maintained globally through the close association between domestic dogs and cattle farms.4 Evidence also exists for a sylvatic cycle, involving wild canids and ruminant species in North America, with a particular high seroprevalence noted among North American white-tailed deer.4
The N. caninum life cycle involves three main life stages: sporozoites within sporulated oocysts, rapidly dividing tachyzoites, and slowly dividing bradyzoites that are sequestered from the host immune system within tissue cysts.4 The oocyst is the environmentally hearty form of the parasite and is formed by sexual replication in the intestinal epithelial cells of canids and subsequently expelled in their feces. The oocysts then sporulate within 24-72 hours in the environment and develop two sporocysts, each of which contains four sporozoites.4 Intermediate hosts become infected by ingesting sporulated oocysts, which then release their sporozoites in the gastrointestinal tract. Sporozoites then infect intestinal epithelial cells, where they transform into tachyzoites that infect a variety of nucleated cells, including mononuclear cells that can traffic them throughout the body, and then replicate within parasitophorous vacuoles within the host cell cytoplasm.4
During acute infection, tachyzoites may be found in almost all tissues of the body and repeated cycles of replication, host cell lysis, tachyzoite release, and infection of surrounding cells produce characteristic lesions and clinical signs. After approximately 20 cycles of replication, tachyzoites differentiate into bradyzoites within tissue cysts under pressure from the host immune response, and a quiescent, asymptomatic infection develops that can last for the life of the host.4 This long-term persistent infection may recrudesce with changes in the host’s immune status, such as pregnancy, immunodeficiency, or, as in this case, immunosuppressive therapy.4
Transmission of N. caninum, which can be vertical or horizontal, has been extensively studied due to the economic losses caused by abortions in infected cattle herds.3,4 Vertical transmission is the predominant form of transmission in cattle, and can occur following ingestion of sporulated oocysts from the environment (exogenous transplacental transmission) or following recrudescence of infection of a persistently infected cow during pregnancy (endogenous transplacental transmission).4 Endogenous transplacental transmission is the main mechanism by which N. caninum is maintained in the domestic cattle population, with fetal transmission rates as high as 95%.4 Persistently infected cattle have an abortion risk between 1.7-7.4 times the risk of a naïve cow, though the risk diminishes with successive pregnancies, suggesting that some degree of host immunity sufficient to frustrate endogenous transplacental transmission develops over time.4
As illustrated by this case, a robust host immune response is critical to controlling N. caninum infection. Studies in cattle demonstrate that a Th1-type response is essential for restricting parasite replication and the conversion of tachyzoites to bradyzoites in tissue cysts.4 Conversely, host immunosuppressiontips the scales toward a Th2-type response, causing recrudescence characterized by the conversion of bradyzoites to tachyzoites and uncontrolled tachyzoite proliferation.4
The role of humoral immunity is less clear, but it likely controls N. caninum infection by antibody neutralization of extracellular tachyzoites.4
This week’s moderator was Dr. Francisco Uzal, Professor in the Department of Pathology, Microbiology and Immunology at the University of California Davis School of Veterinary Medicine and the California Animal Health and Food Safety Laboratory. Discussion of this case initially centered on the terms “ulceration” and “erosion,” and whether either was appropriate to describe the mucosa in this case. Conference participants opined that the difference between the two was a matter of depth, with some participants using “ulceration” to indicate that the mucosal epithelium is lost, whereas others define the term to mean a lesion that extends deep to the muscularis mucosa. The term “erosion,” for both camps, is reserved for more superficial mucosal loss, especially in areas in which the mucosa is composed of multiple layers of cells, such as the esophagus.
Participants next discussed the significant vasculitis observed throughout the section and whether the vasculitis was the cause or an effect of the massive, transmural necrosis present throughout the section. Participants felt that, while organisms were found in many endothelial cells, the vasculature was largely caught up in the inflammatory and necrotic milieu produced by N. caninum. Participants also noted the clinical history of inflammatory bowel disease and wondered if pathology associated with that condition was confounding histologic interpretation.
Discussion of the morphologic diagnosis was relatively straightforward in this case, though participants felt they would not have been able to determine whether the section was from the large or small intestine without the contributor’s tissue identification. Participants also felt that the vasculitis and the tissue distribution of the organisms were notable histologic features and were thus included in the morphologic diagnosis.
References:
- Barber JS, Trees AJ. Clinical aspects of 27 cases of neosporosis in dogs. Vet Rec. 1996;139(18):439-443.
- Brown CB, Baker DC, Barker IK, 2007. The alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 5th ed. Vol 2. 2007; Elsevier Saunders: 272-273.
- Buxton D, McAllister MM, Dubey JP. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002;18(12):546-552.
- Donahoe SL, Lindsay SA, Krockenberger M, Phalen D, Slapeta J. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int J Parasitol Parasites Wildl. 2015; 4(2):216-238.
- Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol. 2003;41(1):1-16.
- Dubey JP, Schares G. Diagnosis of bovine neosporosis. Vet Parasitol. 2006;140(1-2):1-34.
- Dubey JP, Schares G, Ortega-Mora LM, Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev. 2007;20(2):323-367.
- Dubey JP, Jenkins MC, Rajendran C, Miska K, Ferreira LR, Martins J, et al. Gray wolf (Canis lupus) is a natural definitive host for Neospora caninum. Vet Parasitol. 2011;181(2-4):382-387.
- Hoon-Hanks LL, Regan D, Dubey JP, Porter MC, Duncan CG. Hepatic neosporosis in a dog treated for pemphigus foliaceus. J Vet Diagn Invest. 2013;25(6):807-810.
- La Perle, KM, Del Piero F, Carr RF, Harris C, Stromberg PC. Cutaneous neosporosis in two adult dogs on chronic immunosuppressive therapy. J Vet Diagn Invest. 2001; 13(3):252-255.
- Magaña A, Sánchez F, Villa K, Rivera L, Morales E. Systemic neosporosis in a dog treated for immune-mediated thrombocytopenia and hemolytic anemia. Vet Clin Pathol. 2015;44(4):592-596.
- Reichel MP, Ellis JT, Dubey JP. Neosporosis and hammondiosis in dogs. J Small Anim Pract. 2007;48(6):308-312.
- Ruehlmann D, Podell M, Oglesbee M, Dubey JP. Canine neosporosis: a case report and literature review. J Am Anim Hosp Assoc. 1995;31(2):174-183.
- Schlafer DH, Miller RB. Female genital system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 5th ed. Vol 3. 2007; Elsevier Saunders:514–516.