WSC 22-23
Conference 9
Case III
Signalment:
10-year-old, female, Shiba dog, Canis lupus familiaris.
History:
In echo examination at regular checkup, enlarged gall bladder and extrahepatic bile duct enlargement was found. Computed tomography revealed a 1cm-sized mass proximal to the common bile duct. The mass was surgically removed by blunt dissection.
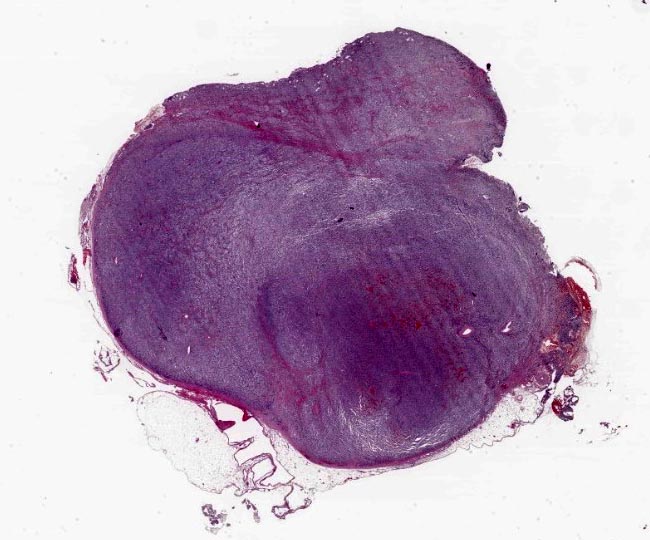
Gross Pathology:
The rounded mass had about 1 cm in diameter, well defined, with a firm consistency and a red brown color.
Laboratory Results:
None reported.
Microscopic Description:
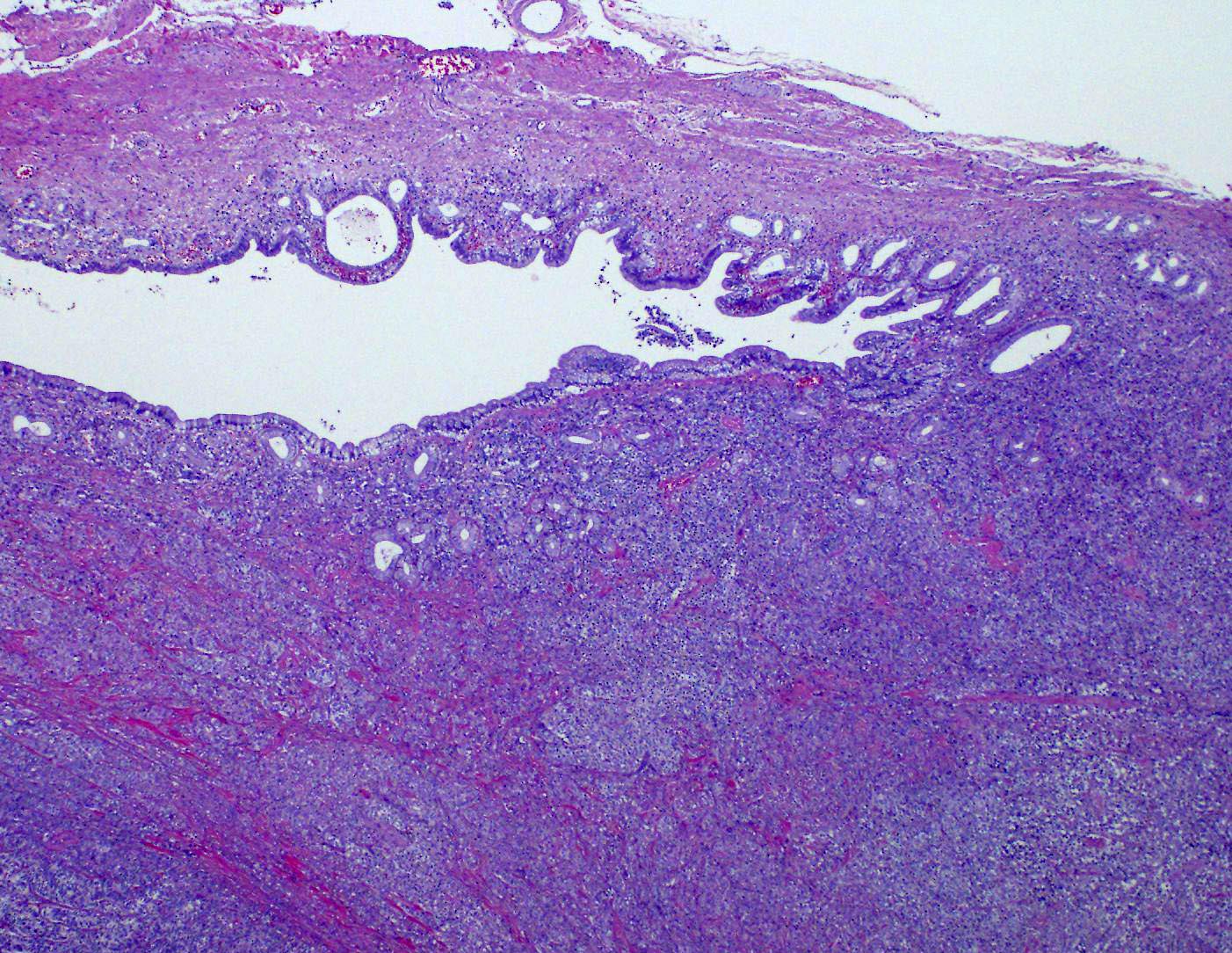
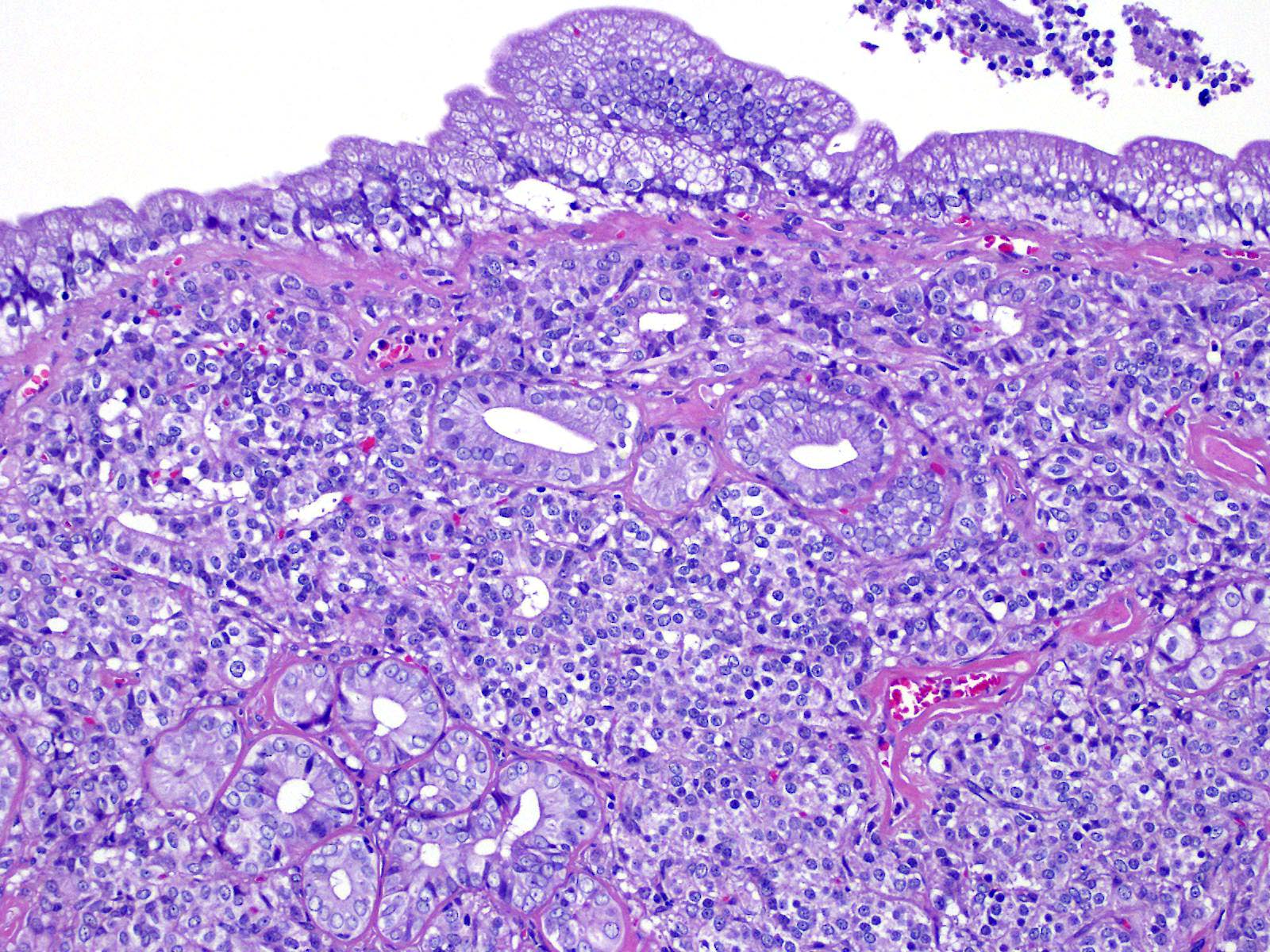
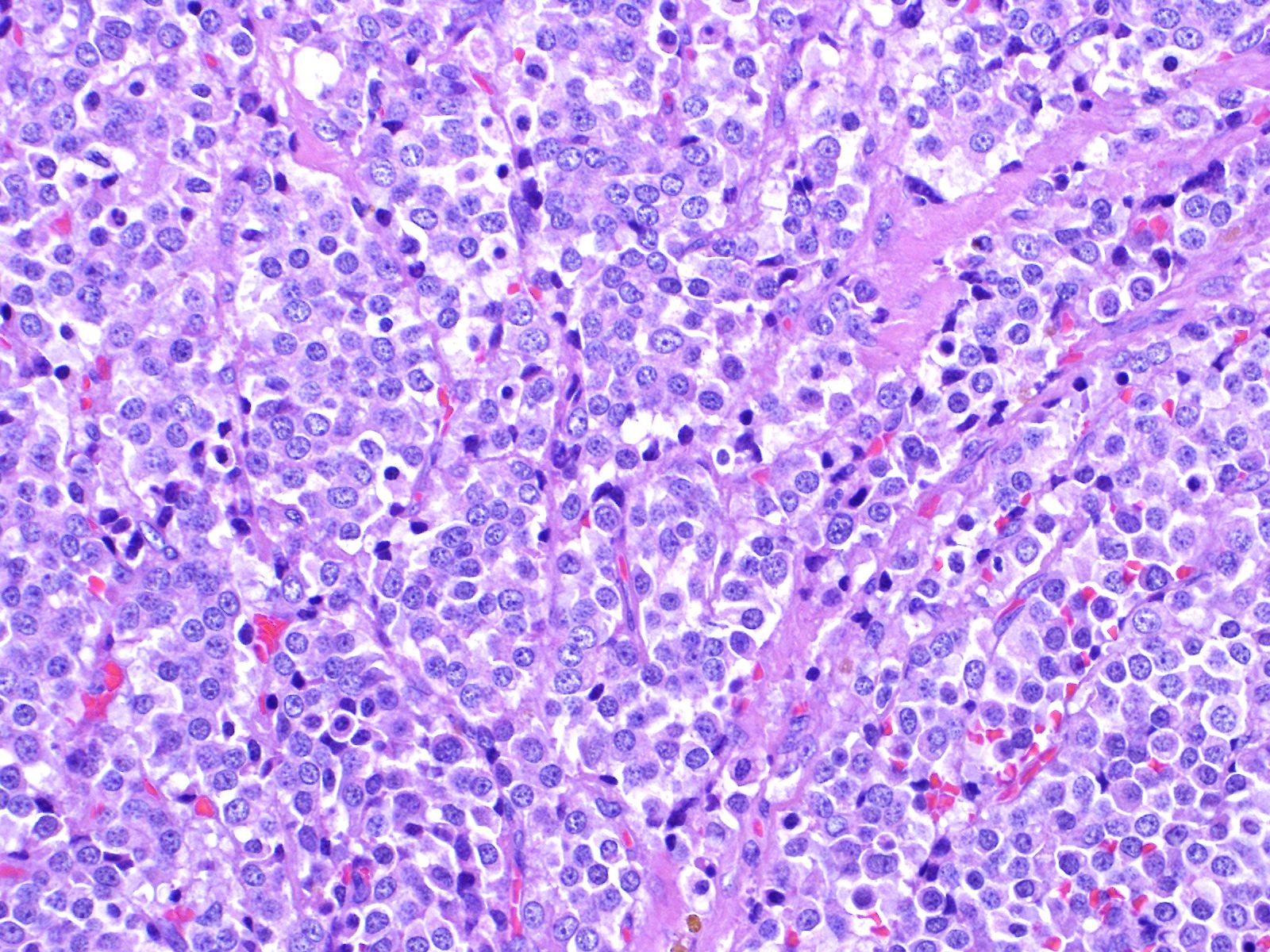
A tumor formed beneath the luminal epithelium of the common bile duct. Tumor cells formed continuously from small diverticula and invaginations of the mucosa beneath luminal epithelium of the common bile duct. A partial demarcated, encapsulated, and infiltrative tumor composed of nests, cords, acinar, and trabecular of epithelial cells supported by fibrovascular stroma. The tumor cells were polygonal, round to small spindle with distinct cell borders. Cells have moderate amounts of a finely granular, lightly eosinophilic cytoplasm, and round to oval, basilar nuclei with finely stippled chromatin and one small nucleolus. Mitoses were rare (2 per 10 HPF, 400X magnification). There was vascular invasion, but no evidence of necrosis.
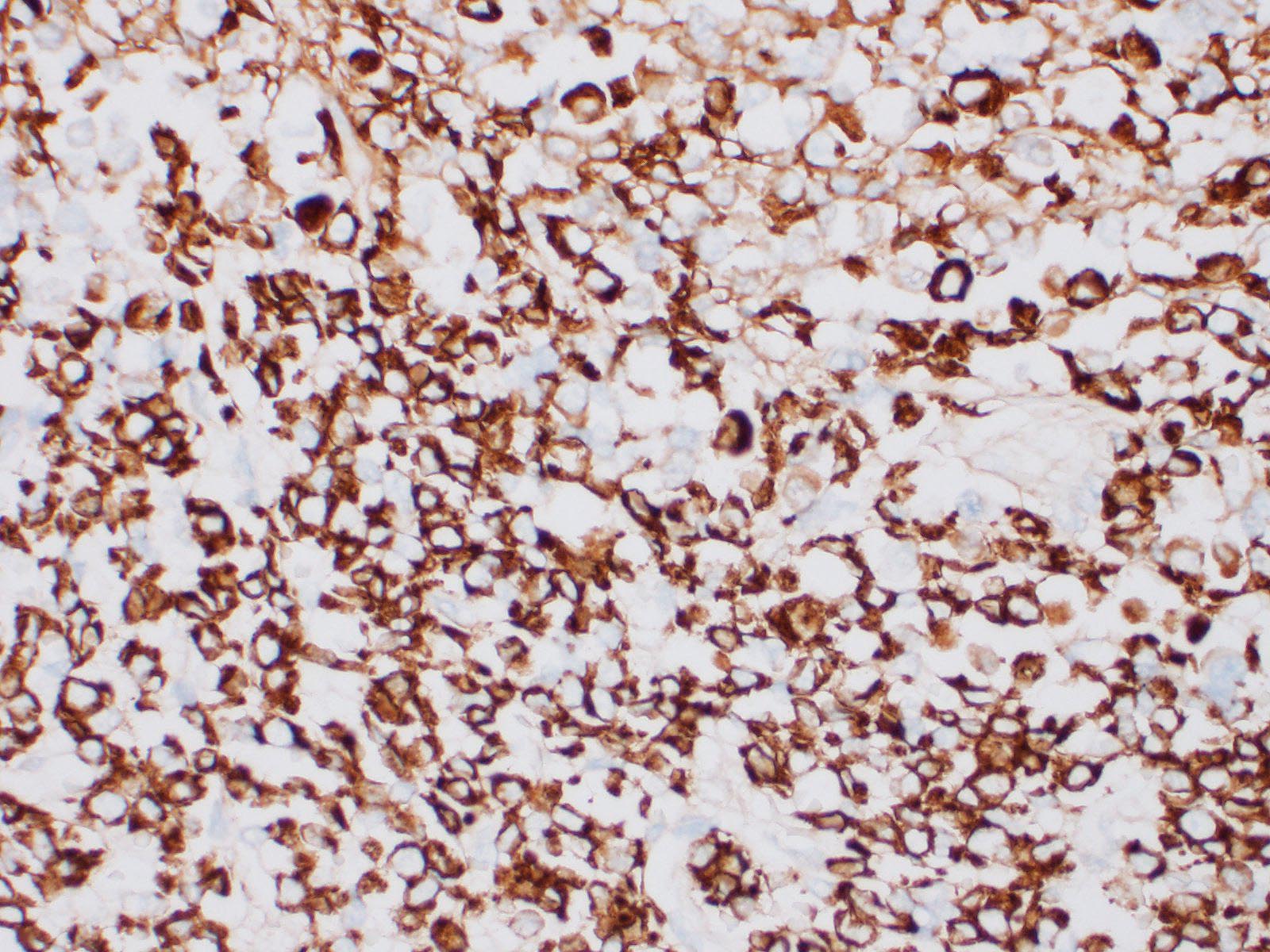
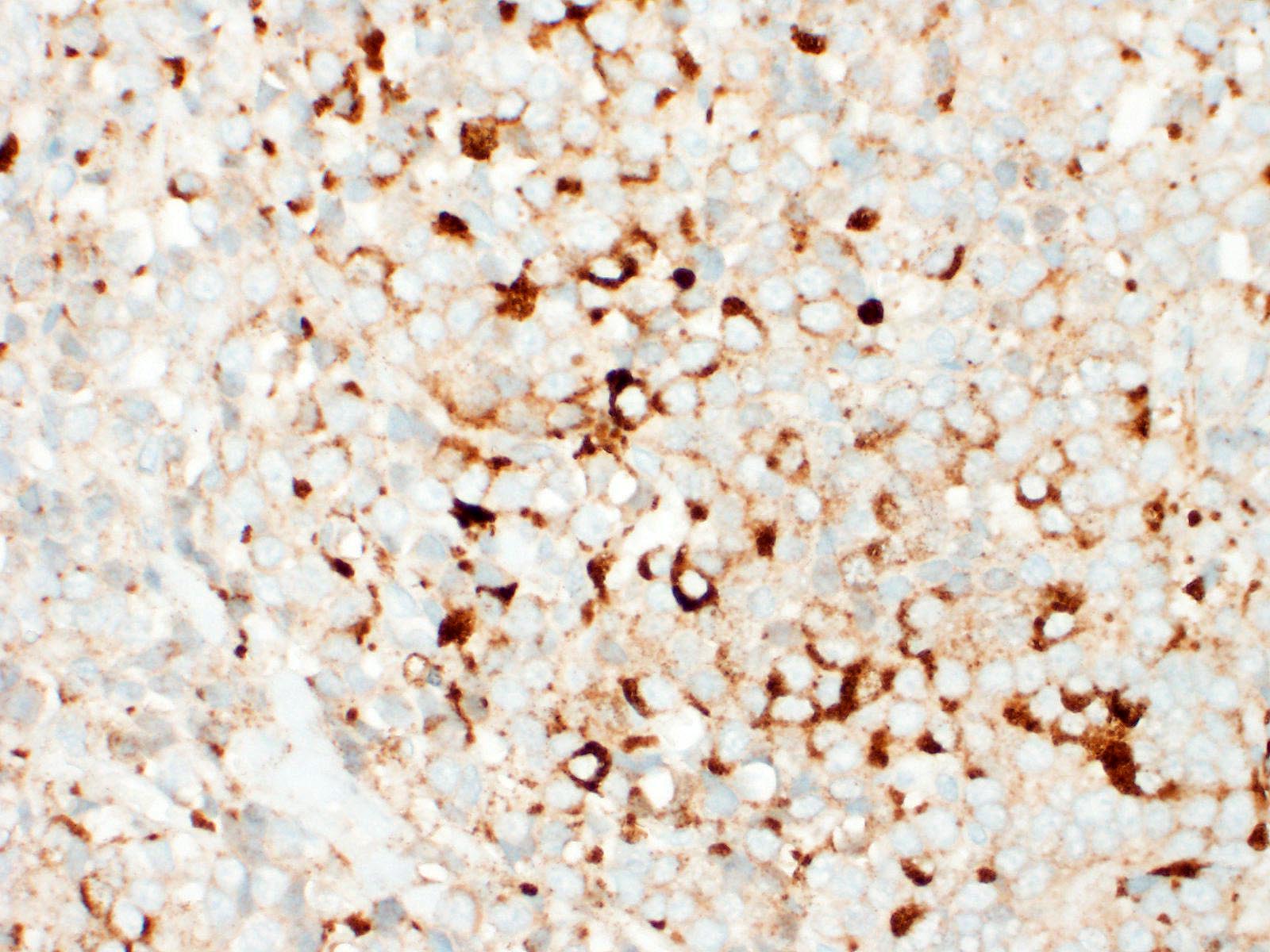
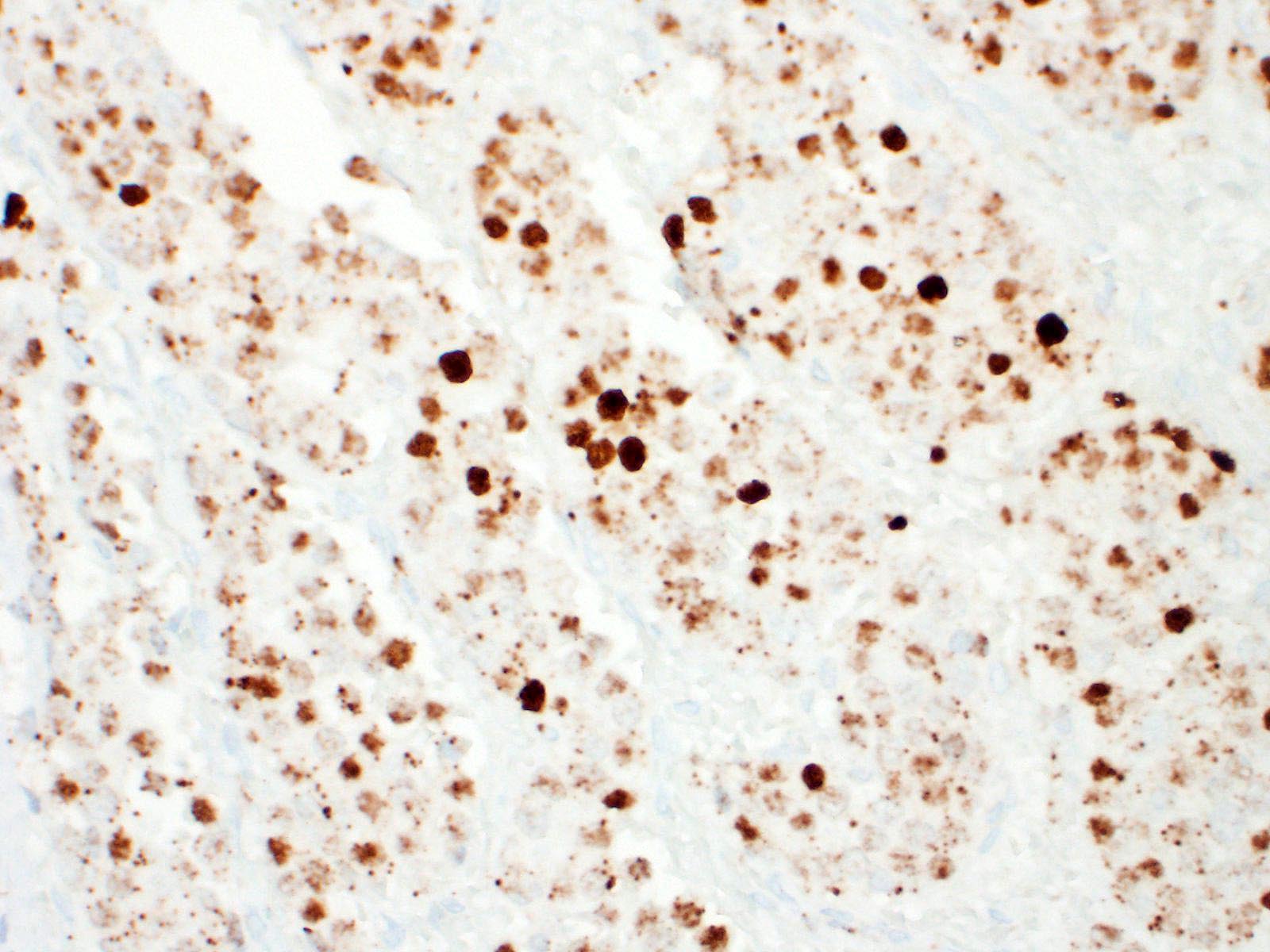
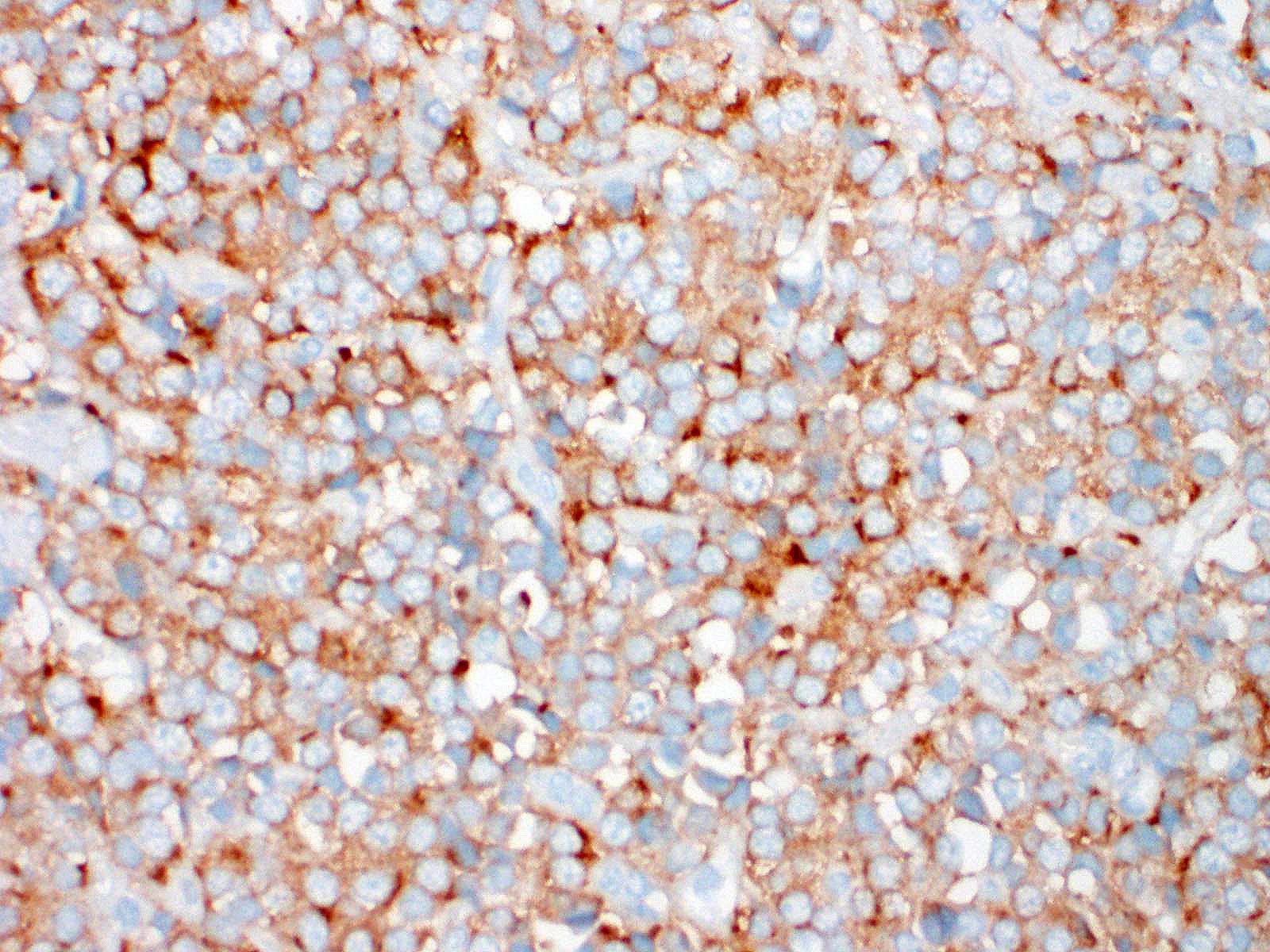
Immunohistochemical investigation showed tumor cells strongly positive for neuroendocrine markers (synaptophysin, Insulinoma-associated1(INSM1)) and cytokeratin (AE1/AE3, CK19). Chromogranin A was negative.
Contributor’s Morphologic Diagnoses:
Extrahepatic bile duct: Carcinoid (neuroendocrine tumor)
Contributor’s Comment:
Carcinoid tumors are thought to arise from embryonal neural crest cells, or so-called argentaffin cells (Kulchitsky cells), which migrate to sites within the respiratory and gastrointestinal tracts during neonatal development. Most cases of neuroendocrine tumors have been reported in the gastrointestinal tract, the respiratory tract, liver, pancreas and central or peripheral nervous system. It has also been confirmed to occur in the skin. In humans, extrahepatic bile duct carcinoid is extremely rare and accounts for 0.1-0.21% of all carcinoids of the gastrointestinal tract, with most reported cases arising from within the gallbladder.1,5 In domestic animals, primary gallbladder neuroendocrine carcinomas of cats, dog, and cow are reported in only a few cases.2,6,8?To the best of our knowledge, there are no reports of primary extrahepatic bile duct carcinoid in dogs.
An important differential diagnosis was cholangiocarcinoma and metastatic tumor. The definitive diagnosis of neuroendocrine tumor is based on histologic features associated with immunohistochemical stainings. A relevant panel including chromogranin A, synaptophysin and NSE is recommended to diagnose hepatic carcinoid.1,5?Recently, INSM1 is a new useful specific marker of neuroendocrine differentiation in neuroendocrine neoplasms. The immunoreactivity for INSM1 was greater than conventional cytoplasmic NE markers (chromogranin A, synaptophysin).1,5 In this case, as INSM1 was strongly diffuse positive, and it became clear that INSM1 can be used as a neuroendocrine marker in dogs. Thus, the morphology of this tumor and the positivity of synaptophysin and INSM1 can eliminate cholangiocarcinoma. Direct infiltration by peripheral organs like liver, pancreas and small intestine or metastasis from other organs was eliminated as a possible diagnosis by no adhesion with surrounding organs and by the gross and morphologic characteristics of the tumor.
The clinicopathologic spectrum of neuroendocrine tumors ranges from the benign carcinoid to the aggressive neuroendocrine carcinoma.1,5 The malignancy of neuroendocrine tumors is based on the following: 1) tumor size (>2 cm), 2) invasion into adjacent tissues, 3) invasion beyond the submucosa and into adjacent tissues, 4) presence of necrosis, 5) overt cell atypia with more than two mitotic cells per 10 high-power fields, 6) hormone expression and loss of chromogen immunoreactivity, and 7) nuclear P53 protein accumulation. 1,5 However, biologic behavior of neuroendocrine tumor is difficult to predict on histological features, since anaplasia features and mitotic index are not reliable to determine the malignancy grade. The evidence of tumor invasion into adjacent tissue could reflect criteria of malignancy. So, it is recommended to consider these tumors as potentially malignant. Hepatic carcinoid has been described as aggressive tumor associated with a common metastatic potential to the peritoneum and draining lymph nodes, however it is suggested that in case of gallbladder carcinoid without evidence of intraperitoneal or distant metastasis, the prognosis is considered as good after a surgical treatment. In this case, carcinoid originated in extrahepatic bile duct has not been reported previously in dog, so its biological behavior is unknown.
Contributing Institution:
Laboratory of Pathology, Faculty of Pharmaceutical Sciences, Setsunan University,
45-1 Nagaotohge-cho, Hirakata, Osaka 573-0101, Japan
JPC Diagnosis:
Bile duct: Neuroendocrine tumor (carcinoid).
JPC Comment:
Neuroendocrine tumors were first described in 1907 by Siegfried Oberndorfer, a pathologist at the University of Munich.3 He coined the term “Karzinoide Tumor” (carcinoid) to describe a special cancer of the gastrointestinal system that was typically benign and not related to an adenocarcinoma.3 Subsequent work throughout the 20th century revealed the neuroendocrine nature of the cells, which were ultimately determined to be entodermal in origin.3 It is now recognized that neuroendocrine tumors, though they may have similar immunohistochemical staining properties, are diverse in their morphology, biologic behavior, and hormone content. 3
A recent study described the features of gall bladder neuroendocrine tumors in 13 dogs. Brachycephalic breeds, particularly the Boston terrier, were overrepresented, and the most common presenting complaint was vomiting.7 Six of the animals had evidence of metastasis to the liver, with two others metastasizing to the lung and one to the mesentery.7 Eight animals had vascular invasion, but the vast majority (12) were removed with clean margins.7 The median survival time for the eight patients which died prior to the end of the study was 3.7 years, and the cause of death in 5 of those patients was neuroendocrine carcinoma. 7
In contrast to the fair to good prognosis implied in the previous report of gall bladder neuroendocrine tumors, hepatic neuroendocrine tumors appear to have a very poor prognosis, with a median survival time of 3 days in one study of 10 dogs.8 Hepatic carcinoids typically affect all liver lobes, and in one study from 1981, 14 of 15 cases metastasized, with 13 cases resulting in carcinomatosis and 14 cases of lymph node metastasis.8,9 These findings indicate that hepatic neuroendocrine carcinomas are potentially aggressive neoplasms.
Another recent study demonstrated that neuroendocrine carcinomas are the most common gastric neoplasm in bearded dragons, accounting for 16 of 26 gastric and 51 total gastrointestinal tract neoplasms.4 In an earlier study on bearded dragons, the most common clinical sign was anorexia, and somatostatin was the only immunohistochemical marker expressed in the neoplasms, suggesting they are somatostatinomas.10 In 6 of 10 of these cases, metastasis occurred to other abdominal organs, and 1 metastasized to the lung, indicating aggressive behavior in this species as well.10
References:
- Fujino K, Yasufuku K, Kudoh S, et al. INSM1 is the best marker for the diagnosis of neuroendocrine tumors: comparison with CGA, SYP and CD56. Int J Clin Exp Pathol 2017;10: 5393-5405.
- Johnson LK, Nunez A, Bracegirdle JR, Dwyer JR, Konold T. Neuroendocrine carcinoma of the liver and gallbladder in a cow. J Comp Pathol. 2008;138: 165-168
- Kloppel G. Oberndorfer and His Successers: From Carcinoid to Neuroendocrine Carcinoma. Endocr Pathol. 2007; 18: 141-144.
- LaDouceur EB, Argue A, Garner MM. Alimentary Tract Neoplasia in Captive Bearded Dragons (Pogona spp). J Comp Pathol. 2022; 194: 28-33.
- McHugh KE, Mukhopadhyay S, Doxtader EE, Lanigan C, Allende DS. INSM1 Is a Highly Specific Marker of Neuroendocrine Differentiation in Primary Neoplasms of the Gastrointestinal Tract, Appendix, and Pancreas. Am J Clin Pathol. 2020;153: 811-820.
- Morrell CN, Volk MV, Mankowski JL. A carcinoid tumor in the gallbladder of a dog. Vet Pathol. 2002;39: 756-758.
- O’Brien KM, Bankoff BJ, Rosenstein PK et al. Clinical, histopathologic, and immunohistochemical features of 13 cases of canine gallbladder neuroendocrine carcinoma. Jour Vet Diag Invest. 2021; 33(2): 294-299.
- Patnaik AK, Lieberman PH, Erlandson RA, Antonescu C. Hepatobiliary neuroendocrine carcinoma in cats: a clinicopathologic, immunohistochemical, and ultrastructural study of 17 cases. Vet Pathol. 2005;42: 331-337.
- Patnaik AK, Newman SJ, Scase T, et al. Canine Hepatic Neuroendocrine Carcinoma: An Immunohistochemical and Electron Microscopic Study. Vet Pathol. 2005; 42:140-146.
- Ritter JM, Garner MM, Chilton JA, Jacobson ER, Kiupel M. Gastric Neuroendocrine Carcinomas in Bearded Dragons (Pogona vitticeps). Vet Pathol. 2009; 46:1109-1116.