Signalment:
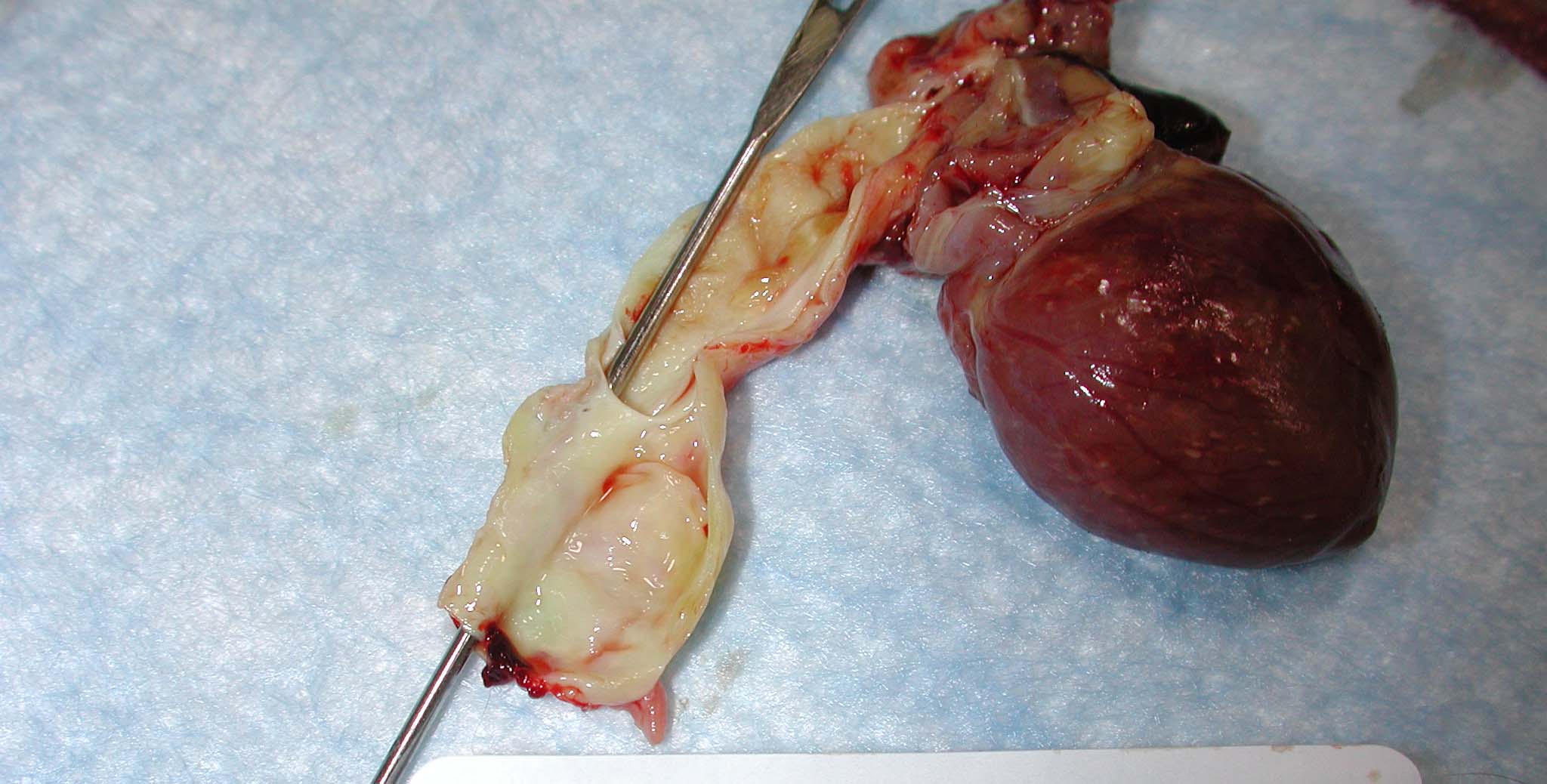
Gross Description:
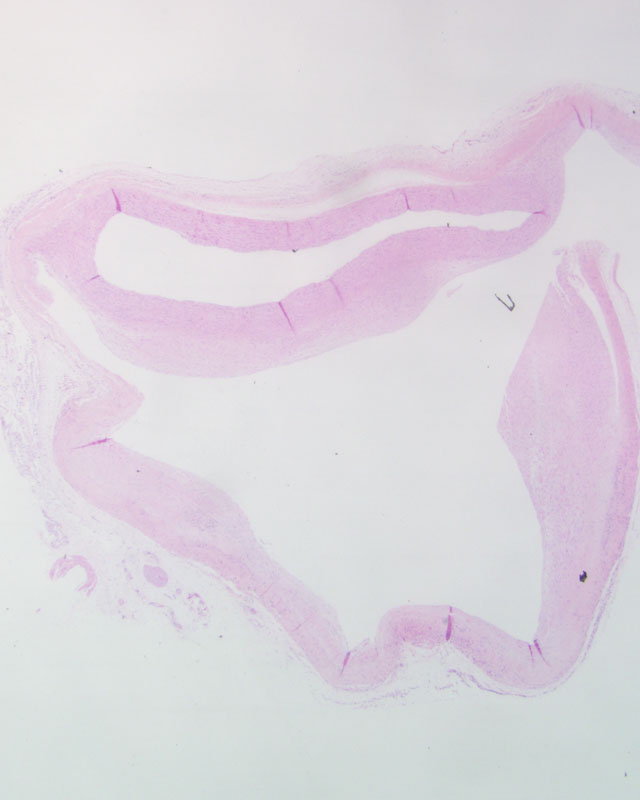
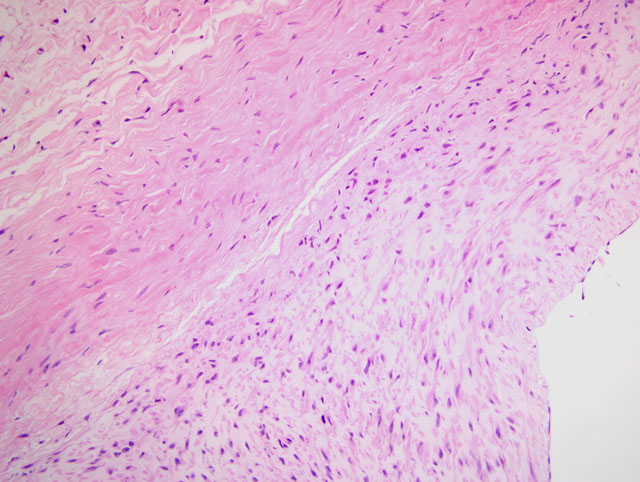
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Hematocrit 19.6% ↓; RBC 2.66 x 106/μl ↓; BUN 45 mg/dl ↑; creatinine 1.4 mg/dl ↑
Condition:
Contributor Comment:
In general, the incidence rates for aortic aneurysms in nonhuman primates appear to be low; however, spontaneous lesions have been reported in the gorilla and squirrel, howler, capuchin, patas, spider, and owl monkeys.(1) In contrast, aortic aneurysms are not uncommon in the owl monkey, with an incidence rate of 8.6% (N=257) in one report. The majority of the aneurysms in this report were classified as dissecting with only three others termed saccular aneurysms. Aortic plaques were seen in some of these animals, as was chronic nephropathy, cardiomegaly, left ventricular hypertrophy, pericardial effusion, pleural effusion, pulmonary edema, hemothorax, hemoperitoneum, hepatomegaly, and cholelithiasis. More females than males were affected.
Hypertension is a major risk factor for aortic dissection in human males aged 40-60 years. Degenerative changes in the tunica media of the aorta may also be important. Inherited connective tissue disorders that lead to abnormal vascular structure (e.g. Marfan syndrome) fall into the latter category. Complications following arterial cannulation and pregnancy have also been associated with aortic dissection in humans. Interestingly, atherosclerosis and medial scarring due to diseases such as syphilis are not usually associated with dissections. Following the particular predisposing factor(s), an intimal tear develops with hemorrhage into the wall of the aorta.(3) Conversely, the initiating event may be a ruptured vasa vasorum with bleeding into media.(5) The pathogenesis of the aortic dissection in this monkey was not determined.
JPC Diagnosis:
Conference Comment:
The most important risk factor for aortic dissection in humans is hypertension; less commonly, aortic dissection is associated with abnormal vascular extracellular matrix (ECM) due to inherited or acquired connective tissue disorders.(3) Participants reviewed one such disorder, Marfan syndrome, an inherited defect in the extracellular glycoprotein fibrillin-1 which results from a mutation in the FBN1 gene. Fibrillin-1 is a major component of the ECM microfibrils that provide the scaffolding on which tropoelastin is deposited to form elastic fibers. Fibrillin-1 abnormalities result in defective mechanical properties of the ECM in the cardiovascular system and eyes, resulting in aortic aneurysm, aortic dissection, and lens subluxation or dislocation. Moreover, since normal microfibrils sequester transforming growth factor β (TGF-β) and control its bioavailability, Marfan syndrome is characterized by excessive activation of TGF-β; this not only further contributes to the altered vascular ECM integrity, but likely accounts for other clinical manifestations of the syndrome not attributable to ECM abnormalities (e.g. bone overgrowth).(2) These observations are supported by studies in a mouse model of Marfan syndrome in which Fbn1 +/- mice developed myxomatous mitral valve and aortic lesions that were prevented by the administration of TGF-β antibodies, demonstrating the importance of TGF-β in the pathogenesis of the lesions and indicating that elevated TGF-β may be primarily responsible for the development of mitral valve prolapse in children with Marfan syndrome.(4)
While in most cases of aortic dissection no specific underlying pathology is identified in the aortic wall, the most frequently detected lesion is cystic medial degeneration in the absence of inflammation. In this case, some participants noted areas of cystic medial degeneration and therefore considered, in addition to Marfan syndrome, other causes of vascular ECM abnormalities, including Ehlers-Danlos syndrome, vitamin C deficiency, and defects in copper metabolism.(3) Some participants noted the presence of foam cells and rare cholesterol clefts, reminiscent of atherosclerosis; however, their subadventitial location is not consistent with atherosclerosis, and as mentioned by the contributor, while atherosclerosis is among the most important predisposing factors for aneurysms (along with hypertension), dissections are unusual in the presence of atherosclerosis, presumably because of medial fibrosis precluding propagation of the dissection.(3) Nevertheless, the gross description of yellow plaques within the aorta is consistent with atherosclerosis and participants could not exclude it as a causal or contributory factor in this case. Finally, some participants noted striking microscopic resemblance to a stented vessel, and dissection can occur iatrogenically due to complicating arterial cannulations.(3)
References:
2. Kumar V, Abbas AK, Fausto N, Aster JC: Genetic disorders. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 144-145. Saunders Elsevier, Philadelphia, PA, 2010
3. Mitchell RN, Schoen FJ: Blood vessels. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 506-510. Saunders Elsevier, Philadelphia, PA, 2010
4. Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC: TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 114:1586-1592, 2004
5. Virmani R, Burke AP: Nonatherosclerotic diseases of the aorta and miscellaneous diseases of the main pulmonary arteries and large veins. In: Cardiovascular Pathology, eds. Silver MD, Gotlieb AI, Schoen FJ, 3rd ed., pp. 109-117, Churchill Livingstone, New York, NY, 2001