Signalment:
Three-year-old ovariohysterectomized
female domestic shorthair
cat (
Felis catus).This cat had a history of tail
chewing, hair loss and recurring dermatitis
over the tail and caudodorsum, occurring since this cat was acquired as a kitten from
Florida. Affected areas of skin were alopecic
with erythema, crusted papules, plaques, and
nodules. There was limited to no clinical
response to treatment with fluoxetine,
topical antibiotics and regular flea
prevention and the cat deteriorated
following injections of depomedrol and
cefovecin. Following biopsy and initial
diagnosis, the cat underwent tail amputation;
however, the cat had recurring dermatitis
over the caudodorsum 3 weeks
postoperatively and was euthanized and
submitted for necropsy.
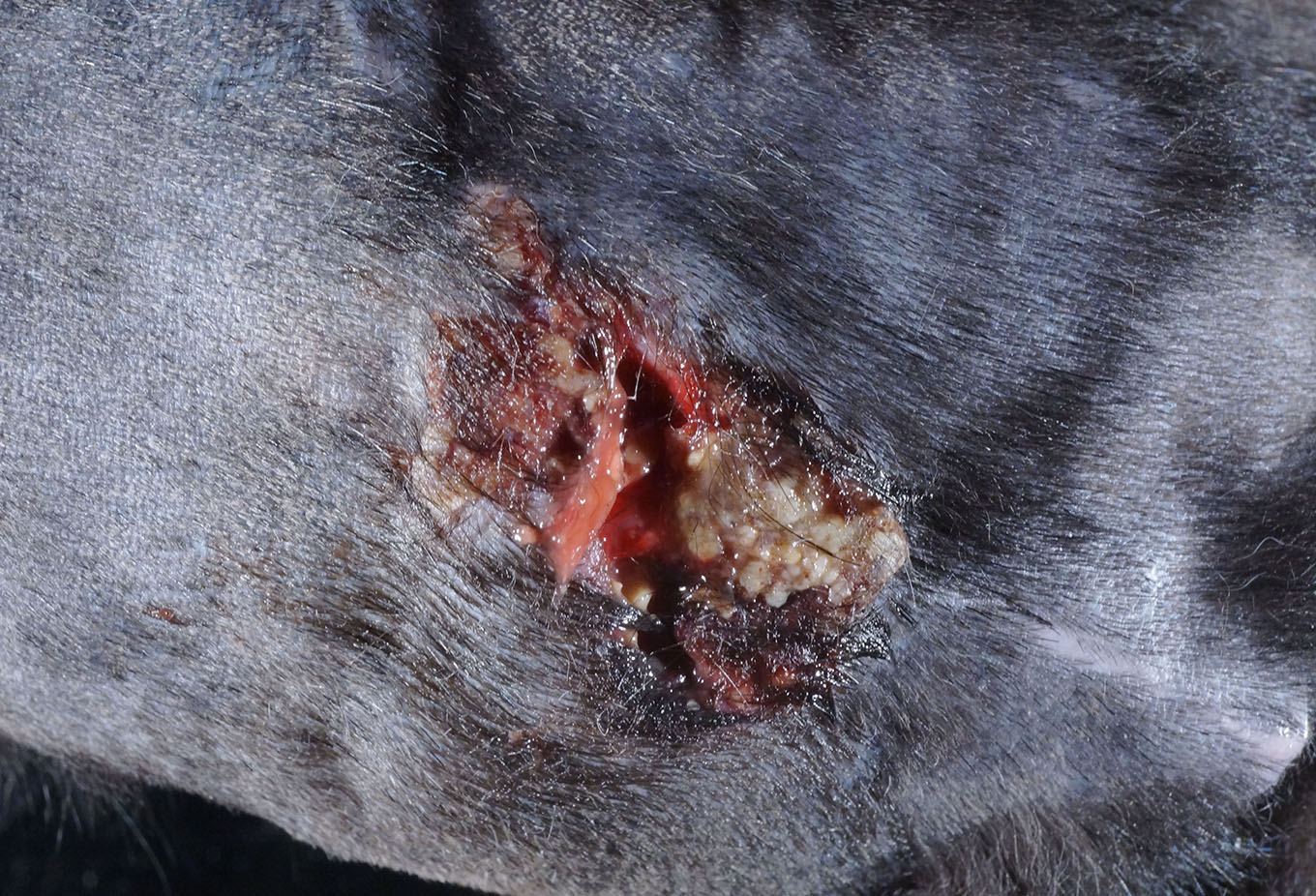
Gross Description:
At necropsy, there were
two reddened, ulcerated areas over the
caudodorsum with variable brown crusts and
dried red-brown exudate. The caudal, larger
area of ulceration and crusting was
overlying a healing scar within the skin
(interpreted as part of the surgical site from
the previous tail amputation). The cranial,
smaller area of ulceration was not associated
with the previous surgical wound. On cut
section, these areas extended into and
expanded the subcutis, with poorly
demarcated, tan to pink, firm, multifocal to
coalescing nodules. There was no gross
involvement of the underlying vertebrae and
no gross evidence of spread to lymph nodes
or visceral organs.
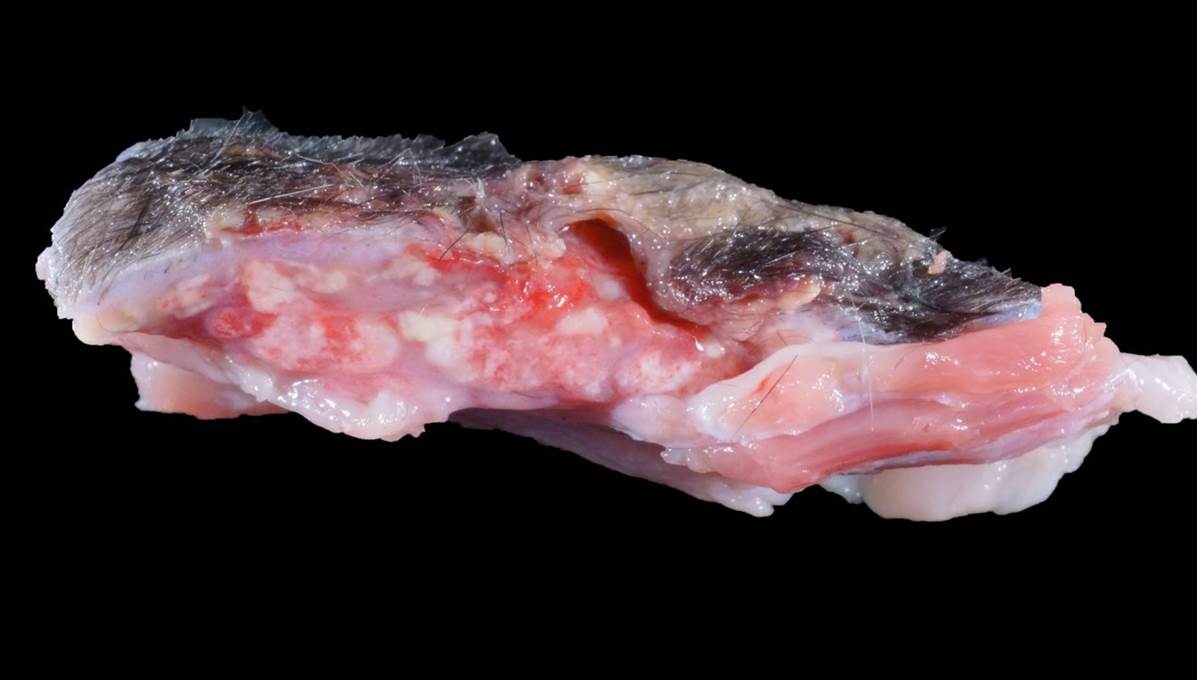
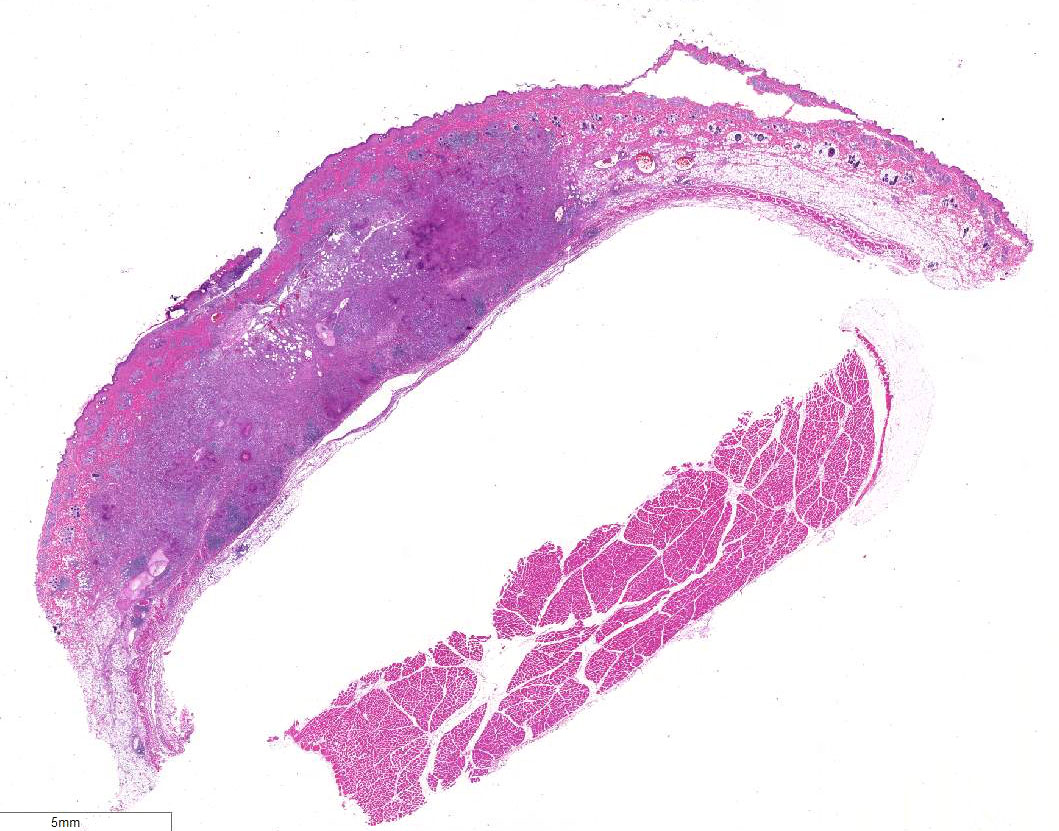
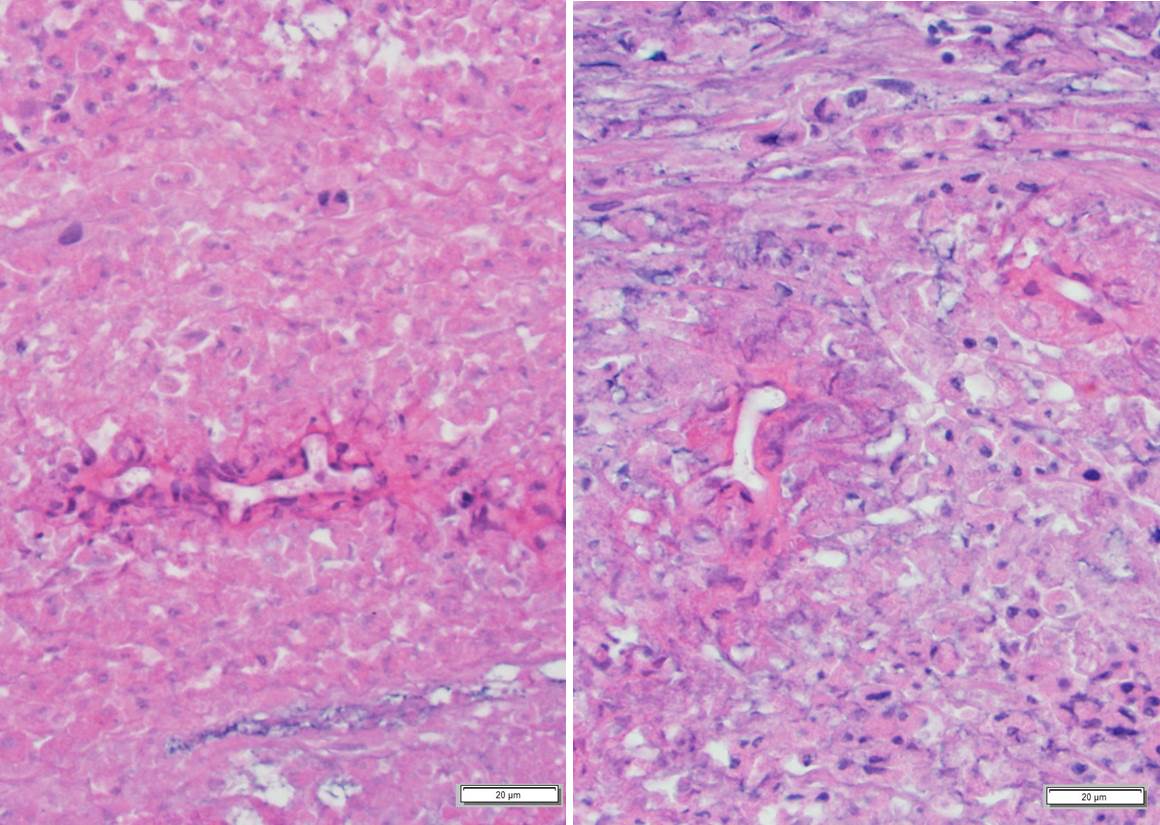
Histopathologic Description:
The section
examined was taken from the cranial,
smaller area of skin ulceration not associated
with the previous surgical wound. Underlying
a locally extensive area of ulcerated
epidermis and expanding the dermis and
subcutis, there is a poorly demarcated, nonencapsulated
infiltration of large numbers of
eosinophils, moderate lymphoplasmacytic
and histiocytic infiltrates and fewer
neutrophils, with extensive, multifocal areas
of eosinophilic necrotic and karyorrhectic
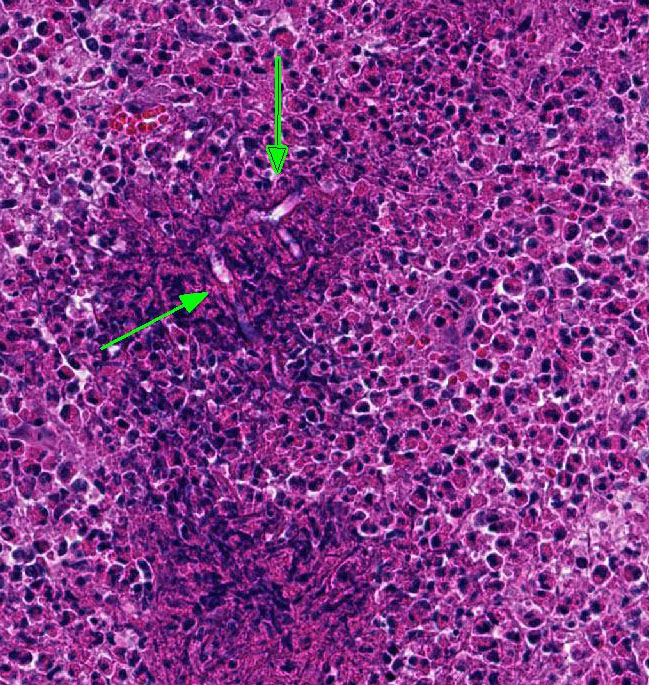
cellular debris. Within areas of necrosis,
there are small to moderate numbers of
predominantly negatively stained, extracellular,
10-15 µm diameter hyphae with
variably prominent round or bulbous
dilatations and intermittent right angle
branching. Areas of necrotic cellular debris
and inflammatory cellular infiltrate surround and isolate small aggregates of irregular,
eosinophilic collagen fibers (collagenolysis).
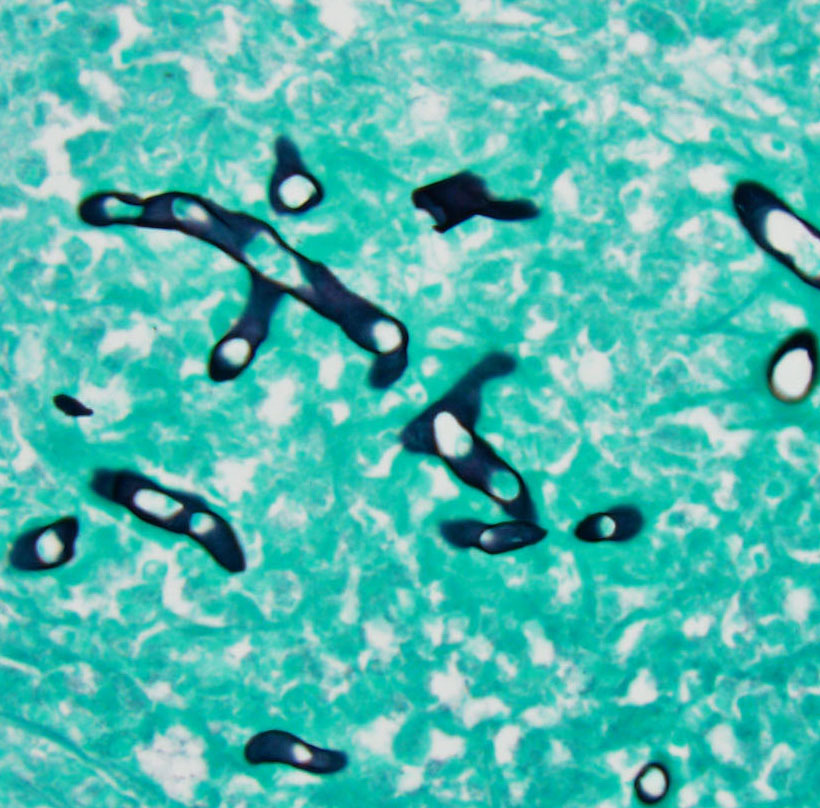
Special Stains: Staining with Grocotts
methenamine silver (GMS) yielded
moderate numbers of broad, thick-walled
hyphae of varying diameters that are
occasionally septate. Staining with PeriodicAcid-Schiff
(PAS) highlights hyphae with
similar morphology, albeit less prominently.
Morphologic Diagnosis:
1. Skin, caudodorsum: Dermatitis,
panniculitis, steatitis and myositis,
eosinophilic and histiocytic, with lymphoplasmacytic
infiltrates, marked, locally
extensive, chronic with multifocal necrosis
and hyphae
2. Skin, caudodorsum: Ulceration, multifocal,
moderate to marked, chronic
Lab Results:
Previous biopsy and
culture of the affected areas yielded scant
mold; further identified as
Lagenidium sp.
by 18S/ITS (internal transcribed spacer)
nucleic acid amplification and sequence
analysis. Culture of necropsy samples was
unable to repeat isolation of
Lagenidium
spp.
Condition:
Fungal dermatitis/Lagenidium sp.
Contributor Comment:
The fungal
hyphae present in this case are consistent
with a recurrence of dermatitis associated
with Lagenidium sp., previously confirmed
in this cat by 18S/ITS nucleic acid
amplification and sequence analysis.
Members of the genus
Lagenidium sp. are a
group of the
Oomycetes, closely related to
Pythium sp. and often referred to as water
molds.
Oomycetes are frequently pathogens
of plants, nematodes, and insect larvae; however, they are occasionally associated
with disease in mammals.
2 The most widely
known Lagenidium species is Lagenidium
giganteum, a pathogen of mosquito larvae
that has previously been implemented as a
biologic control agent.
2 Although stages of
oomycetes may be morphologically similar
to fungal hyphae, oomycetes are
phylogenetically distinct from fungi.
7,8 In
contrast to fungi, the cell wall of oomycetes
contains cellulose and β-glucan rather than
chitin
8
, and the cell membranes lack
ergosterol.
7 Oomycetes also differ from
fungi with respect to life stages produced,
including the production of sporangia and
biflagellate zoospores.
7
Most case reports of lagenidiosis involve
dogs; however, there have been several
recent reports in cats.
7 Infection with
Lagenidium sp. in dogs typically occurs in
young to middle-aged dogs and most
frequently occurs in southeastern United
States.
2,3,7 Exposure to water bodies such as
lakes and ponds is frequently, but not
always, reported.
3 Recent molecular work
has led to the description of two closely related pathogens in dogs; Lagenidium
giganteum forma caninum and
Paralagenidium
karlingii.
5 Lagenidium
giganteum forma caninum causes cutaneous
or subcutaneous disease with frequent widespread
dissemination, involving visceral
organs, lymph nodes and/or great vessels.3
Paralagenidium karlingii infection in dogs
results in chronic ulcerative and/or nodular
dermatitis that does not typically
disseminate.
5 Recommended treatment for
lagenidiosis is wide surgical resection where
possible.
1,3,7 Prognosis is poor for
disseminated disease, with lagenidiosis
poorly responsive to medical therapy.
2,9
Differential diagnoses for lagenidiosis on
clinical presentation and histopathology may
include pythiosis, resulting from infection
with the oomycete, Pythium insidiosum; and
zygomycosis, involving infection with
fungal organisms such as Basidiobolus
ranarum and Conidiobolus spp..
1,2,5 All may
result in granulomatous and/or eosinophilic
inflammation and morphologically appear as broad, irregular branching hyphae that are
rarely to occasionally septate.
1
Differentiation is clinically important due to
differing treatment and prognosis
7,8.
Diagnosis of lagenidiosis can be
challenging. Cytology and histopathology
yield hyphae with similar morphology to
pythiosis and zygomycosis.4 Although there
may be subtle differences in size and/or
morphology of hyphae, histopathology
cannot be used for definitive differentiation.
3
Fungal culture is possible but can be
difficult due to the fastidious nature of
Lagenidium sp. (particularly the sexual
stages). Definitive diagnosis requires
confirmation through molecular assays on
tissue or cultured isolates.
3,7,9 Lagenidium
sp. should be considered a differential in
cats with granulomatous to eosinophilic,
nodular to ulcerative dermatitis.
JPC Diagnosis:
Haired skin and subcutis:
Dermatitis, panniculitis and myositis,
eosinophilic and gran-ulomatous, and
eosinophilic, focally
extensive, marked, with
multifocal necrosis,
ulceration, and fungal
hyphae, domestic shorthair,
Felis catus.
Conference Comment:
The
contributor provides an
excellent example and
overview of the pathogenic
Oomycete water mold,
Lagenidium sp. As mentioned
above, Lagenidium sp.
is strikingly similar in
geographic distribution,
clinical, and histologic
appearance to the more
commonly diagnosed
Oomycete, Pythium
insidiosum.
6 As a result, the majority of conference participants had
pythiosis as their top differential for this
lesion.
Infection with both Pythium insidiosum and
Lagenidium spp. typically, but not always,
occurs when the host has prolonged contact
with standing or stagnant water containing
the motile aquatic flagellate zoo-spores.
2,3,6
This infectious form of the organism is
attracted by animal fur, damaged skin, and
intestinal mucosa. As a result of contact with
standing water, infections in domestic
animals are most commonly reported in the
limbs, ventral thorax, and abdomen. When a
mammalian host with a skin injury enters a
contaminated pond, the oomycete zoospores
of will encyst upon contact with the injured
skin and mechanically penetrate the tissue,
causing clinical disease.
6
Like pythiosis, this disease is typically
highly aggressive and lesions in the great vessels, mediastinum, lungs, and esophagus
have been reported in dogs. However, unlike pythiosis, gastrointestinal disease has not
been reported in Lagenidium spp.
6Both
entities are associated with a poor to grave
prognosis even with wide surgical excision
of cutaneous masses because the majority of
animals infected with this pathogen have
occult, non-resectable, disease in regional
lymph nodes or distant sites when initially
diagnosed.
2,3 In dogs infected with the less
aggressive species, Paralagenidium
karlingii mentioned by the contributor,
surgery that achieves three cm margins is
often curative.
7 Medical therapy for
lagenidiosis is typically ineffective because
ergosterol, the target for most antifungal
drugs, is lacking in the Oomycete cell
membrane.
6,7
Conference participants discussed this lesion
as a great example of chronic-active
inflammation. Chronic-active inflammation
occurs when the inciting inflammatory
stimulus has not been removed from the
chronic inflammatory process and continues
to elicit an acute inflammatory response.
1References:1. Ackermann M. Inflammation and
healing. In: McGavin MD, Zachary
JF, eds.
Pathologic Basis of
Veterinary Disease. 4th ed. St.
Louis, MO:Mosby Elsevier;
2012:127.
2. Grooters AM. Pythiosis, lagenidiosis
and zygomycosis.
Vet Clin Small
Anim. 2003; 33:695-720.
3. Grooters AM, Hodgin EC, Bauer
RW, Detrisac CJ, Znajda NR,
Thomas RC. Clinicopathologic
findings associated with
Lagenidium
spp. infection in 6 dogs: Initial
description of an emerging
oomycosis.
J Vet Intern Med.
2003;17:637-646.
4. Grooters AM, Foil CS.
Miscellaneous fungal infections. In:
Greene CE, ed.
Infectious Diseases
of the Dog and Cat. 4th ed.
Philadelphia, PA: WB Saunders;
2012:681-683.
5. Hartfield JN, Grooters AM, Waite
KJ. Development and evaluation of
an ELISA for the quantification of
anti-
Lagenidium giganteum forma
caninum antibodies in dogs.
J Vet
Intern Med. 2014; 28:1479-1484.
6. Mauldin E, Peters-Kennedy J.
Integumentary system. In: Maxie
MG, ed.
Jubb, Kennedy, and
Palmers Pathology of Domestic
Animals. Vol 1. 6th ed. Philadelphia,
PA:Elsevier; 2016:657-660.
7. Mendoza L, Vilela R. The
Mammalian pathogenic oomycetes.
Curr Fungal Infec Rep. 2013; 7:198-
208.
8. Raffaele S, Kamoun S. Genome
evolution in filamentous plant
pathogens: why bigger can be better.
Nature Reviews Microbiology. 2012;
10:417-430.
9. Znajda NR, Grooters AM, Marsella
R. PCR-based detection of
Pythium
and
Lagenidium DNA in frozen and
ethanol-fixed animal tissues.
Vet
Dermatology. 2002; 13:187-194.