CASE II: 18-6787 (JPC 4116588).
Signalment:
15-year-old, female, Nigerian dwarf goat (Capra aegagrus hircus).
History:
One month history of hematuria, stranguria, polakiuria. By CT there was thickening of the reproductive tract causing compression of the ureters and secondary hyrdoureter and hydronephrosis. Multiple lung nodules bilaterally.
Gross Pathology:
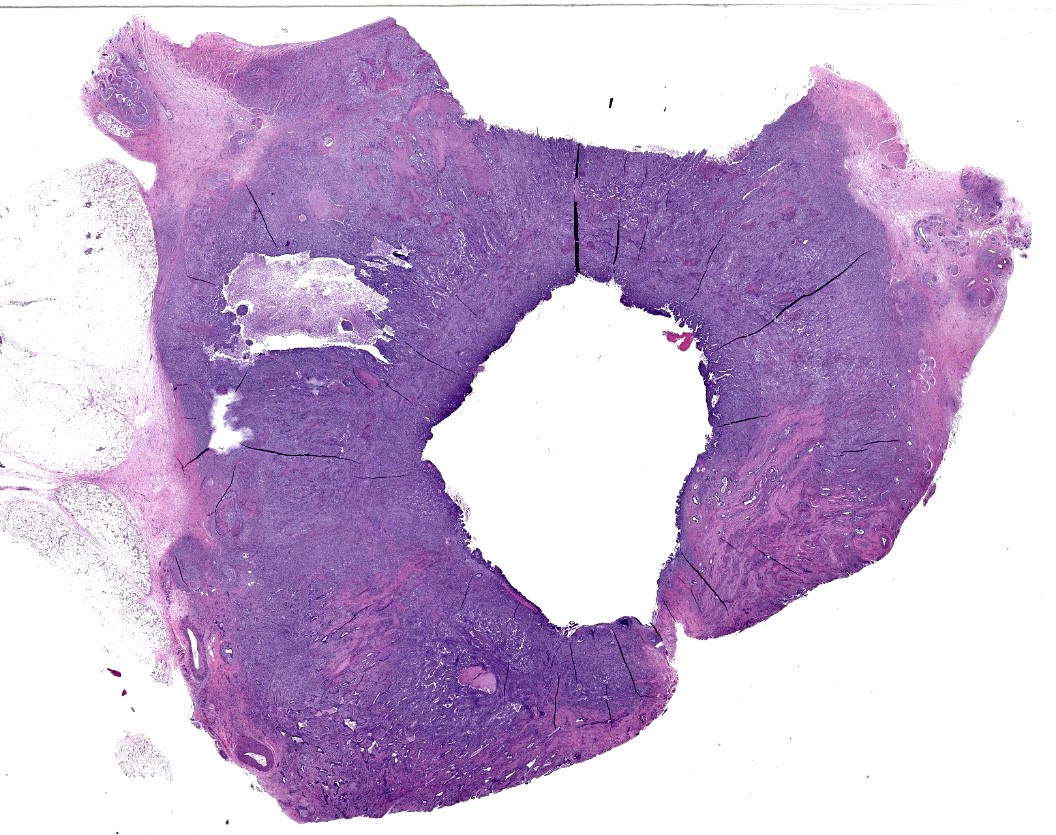
A 26kg Nigerian Dwarf ewe in good body (BCS 3/5) and excellent postmortem condition is necropsied. Conjuctiva and oral mucous membranes are white. The metaphyseal bone marrow from the right femur is white with two small red areas (~3mm) on the lateral aspects. There is a ~5x3cm cystic mass embedded in the fascia between ventral cervical muscles near the thoracic inlet. Both thyroids contain numerous small cysts (~2-3mm in diameter). The lungs contain multifocal semifluctuant white nodules (~1-3cm in diameter) randomly distributed throughout the lung lobes; these nodules contain central areas of necrosis on sectioning. A similar nodular change effaced a tracheobronchial lymph node. The liver contains multifocal firm white areas with irregular margins (~1-3cm in diameter); these foci are solid white on sectioning.
The body of the uterus is firm with multifocal tan, slightly raised nodules on the serosal surface; on cross section, there is annular thickening with necrotic areas and red, mucoid material in the lumen. Both uterine horns contain inspissated pus (pyometra). There are fibrous adhesions between the dorsal aspect of the uterine body and the ventral aspect of the urinary bladder which is moderately distended with clear, yellow urine. The mesovarium and mesometrium is moderately edematous.
The urethra is entrapped in the fibrous adhesions between the bladder and uterus. Both kidneys have moderately dilated pelvises (hydronephrosis).
The right adrenal cortex has a small (~5mm) cyst filled with clear, yellow fluid.
The forestomach is full of fibrous feed. The small intestines contain liquid dark green ingesta. There are formed feces in the distal colon.
Laboratory Results:
None.
Microscopic Description:
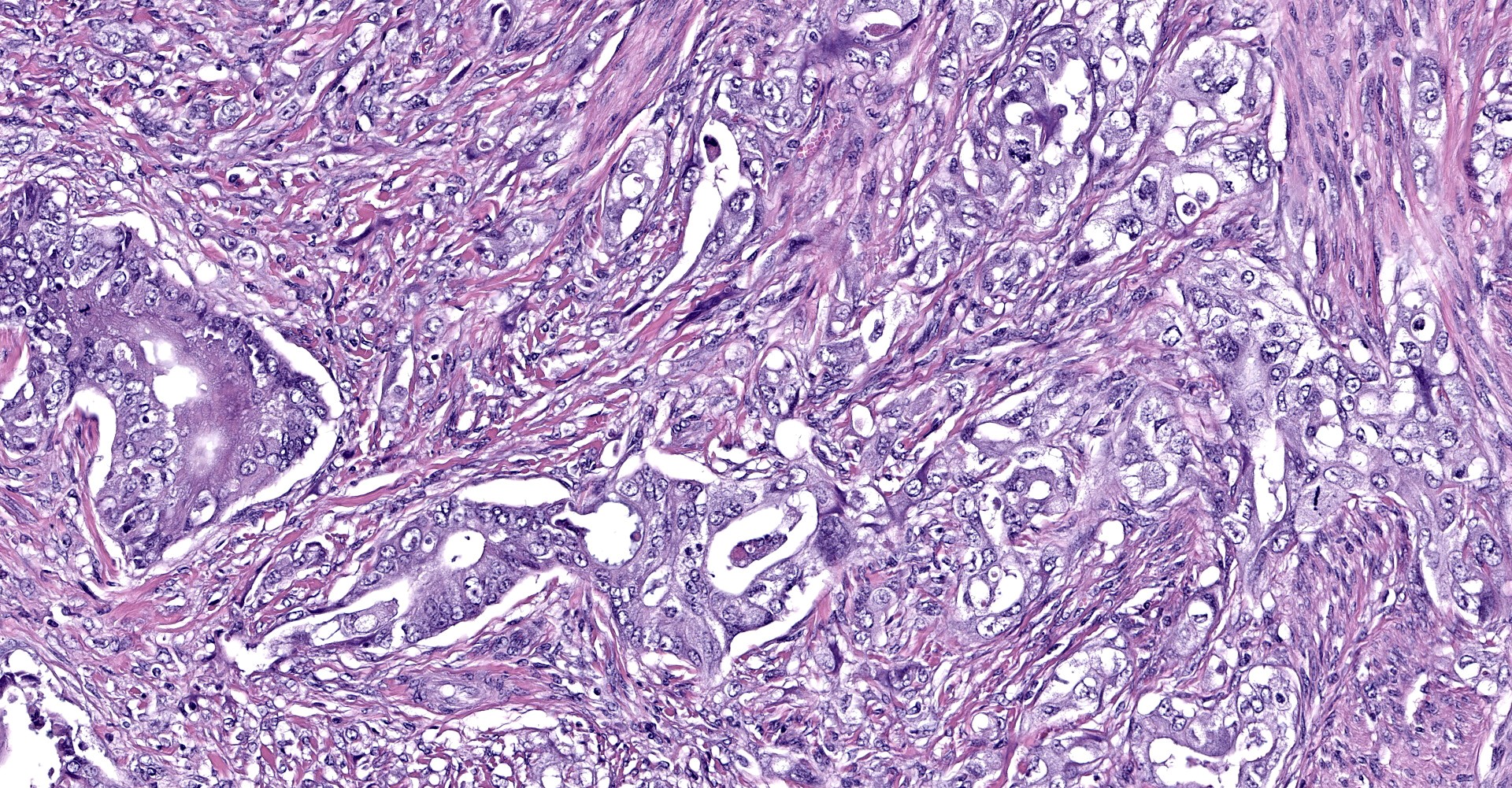
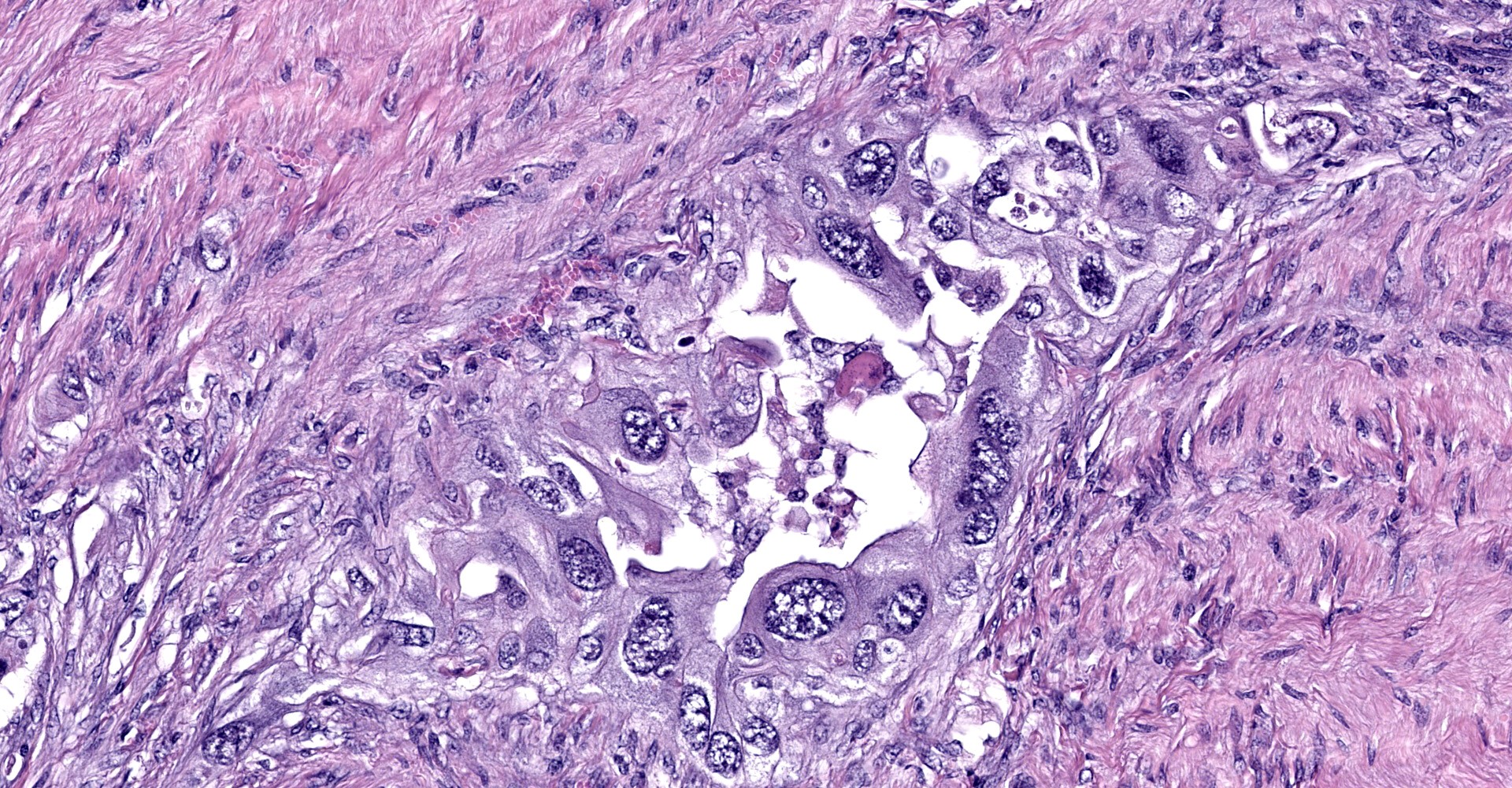
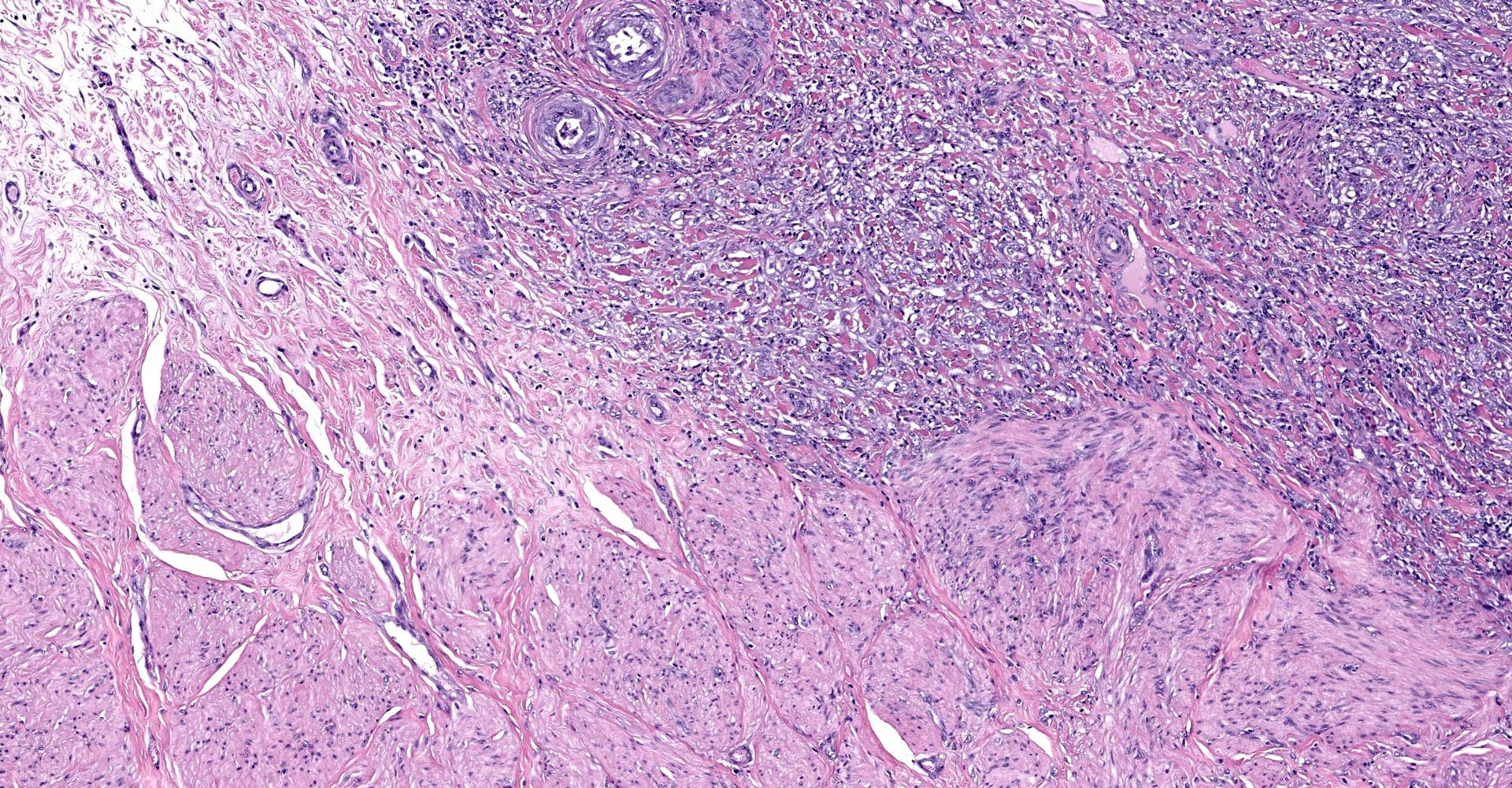
Uterine body - The submucosa-lamina propria is circumferentially infiltrated by neoplastic epithelium forming broad trabeculae and sometimes forming tubular structures surrounded by dense, highly cellular fibrous tissue (desmoplastic response). Neoplastic epithelium exhibits marked anisocytosis and anisokaryosis with regular karyomegaly. They contain round to ovoid, vesiculated nuclei and a small amount of basophilic cytoplasm. In more differentiated areas with tubular formation, the epithelial cells attain a cuboidal to columnar morphology while in less differentiated areas they are rather pleomorphic, ranging from polygonal to spindloid. There are 25 mitotic figures in 10 high power fields. Scattered within the neoplastic population is another cell population that exhibits more pleomorphism, contains a single, large deeply basophilic nucleus with coarsely-clumped chromatin and a small amount of eosinophilic cytoplasm (syncytiotrophoblasts); these cells can also be seen scattered without the surrounding desmoplastic stroma. Neoplastic epithelium and its accompanying desmoplastic response extends through the myometrium and abut the surrounding serosa, enveloping peripheral nerves. Vascular tumor emboli are present at the periphery. There are multifocal areas of lytic necrosis throughout the neoplasm (comprising ~20%) and scattered apoptotic cells within viable tissue. There is mild necrosis of surrounding adipose tissue.
Contributor's
Morphologic Diagnosis:
Uterine ductal
carcinoma, transmural, with vascular invasion and intratumoral
syncytiotrophoblasts (consistent with choriocarcinomatous differentiation).
Other lesions (not submitted):
1. Hepatic and pulmonary metastases.
2. Renal interstitial fibrosis with protein, hyaline and cellular casts.
3. Cystic thymoma (suspect).
Contributor's Comment:
Histopathology confirms the presence of uterine carcinoma, with a tubular pattern predominating. In general, neoplasia is uncommon in goats, with one large case series finding an incidence of 8.7%.6 With regard to the uterus, leiomyomas were most common though case reports of leiomyosarcoma and adenocarcinoma have been documented.1
The striking variance in cellular morphology may reflect transition into what is known as choriocarcinomatous differentiation, which has been documented in both humans and an aged goat.5 In these tumors, there are two distinct neoplastic populations, one which attains the typical ductal epithelium and another which resembles syncytiotrophoblast-like multinucleated giant cells mixed with highly pleomorphic cytotrophoblast-like cells. The former morphology is more consistently found within metastatic lesions in these neoplasms as was seen in this case (metastatic lesions not submitted). The prognostic significance of this type of differentiation is uncertain in caprine species, though expression of embryonic proteins may indicate a more primitive/anaplastic transformation has occurred which potentially behave more aggressively. To confirm this variant we would need to have IHC performed on uterine sections to prove production of chorionic origin antigens, such as chorionic gonadotrophins, within the more pleomorphic cell population.
Other domestic species including rabbits and pigs have been reported to develop choriocarcinomas, neoplasms of trophoblastic origin, without an endometrial component.3,4 These can be subdivided into gestational (i.e. derives from placental tissue during pregnancy) and nongestational (i.e. derives from germ cells within ovarian tissue). Ovarian/nongestational choriocarcinomas have also been documented in non-human primates such as rhesus macaques2 and a cynomolgus monkey.8
Contributing Institution:
Oregon Veterinary Diagnostic Laboratory Oregon State University
Carlson College of Veterinary Medicine
30th and Washington Way
Corvallis, Oregon 97331. https://vetmed.oregonstate.edu/diagnostic
JPC Diagnosis:
Uterus: Uterine adenocarcinoma.
JPC Comment:
Conference attendees were in agreement with the contributor in regard to the diagnosis of uterine adenocarcinoma. Confirmation of choriocarcinomatous differentiation was attempted using a battery of immunohistochemical stains, including human chorionic gonadotrophin (hCG), α-inhibin, and human placental alkaline phosphatase (hPLAP). Unfortunately, immunohistochemical stains for hCG, α-inhibin, hPLAP were non-contributory and choriocarcinomatous differentiation could not be confirmed.
Epithelial neoplasms of the caprine reproductive tract are relatively uncommon. In one study, there were only two reports of uterine adenocarcinoma following a retrospective review of 100 goats with 102 tumors.6 Furthermore, the presentation of uterine adenocarcinoma with pleomorphic cells suggestive of choriocarcinomatous differentiation in this case is exceedingly rare, although a similar case of endometrial adenocarcinoma with choriocarcinomatous differentiation was confirmed in an aged goat, as previously noted by the contributor.5
Choriocarcinoma is a spontaneously occurring, highly malignant, biphasic trophoblastic tumor composed of cytotrophoblastic and syncytiotrophoblastic cellular components and is very rare in both animals and humans.3 As described by the contributor, nongestational choriocarcinomas typically arise within the ovaries; however, nongestational choriocarcinomas can rarely arise from trophoblastic differentiation within poorly differentiated carcinomas, including endometrial carciomas.3 Three hypotheses have been proposed in regard to choriocarcinomatous differentiation in endometrioid adenocarcinoma and are as follows: (1) dedifferentiation of epithelial cells into choriocarcinoma, (2) germ cells that fail to complete migration to the gonads, and (3) multidirectional tumor differentiation from a common stem cell.5
Participants noted the presence of neoplastic cells within not only lymphatics but also nerve ganglia, a feature consistent with perineural invasion. Physically, epineurial, perineurial, and endoneurial spaces of the neuronal sheath are loose connective tissues that can be exploited as low-resistance channels that allow for the rapid migration of neoplastic cells. In humans, perineural invasion has been shown to be a negative prognostic indicator in cases of endocervical adenocarcinoma in addition to other neoplasms.7
References:
1. Dockweiler JC, Cossic B, McDonough SP, et al. Tumor collision of uterine adenocarcinoma and leiomyosarcoma in a goat. J Vet Diagn Invest 2017;29(5):696-699.
2. Farman CA, Benirshcke K, Horner M, Lappin P. Ovarian choriocarcinoma in a Rhesus monkey associated with elevated serum chorionic gonadotropin levels. Vet Pathol. 2005;42(2):226-225.
3. Hirata A, Miyazaki A, Sakai H, et al. Choriocarcinoma-like tumor in a potbellied pig (Sus scrofa). J Vet Diagn Invest. 2014;26(1):163-166.
4. Kaufman-Bart M, Fischer I. Choriocarcinoma with metastasis in a rabbit (Oryctolagus cuniculi). Vet Pathol. 2008;45(1):77-79.
5. Kawashima M, Segawa R, Toshinori Y, et al. Endometrial adenocarcinoma with choriocarcinomatous differentiation in the uterus of a goat. J Vet Med Sci. 2017;79(6):1091-1095.
6. Lohr CV. One hundred and two tumors in 100 goats (1987-2011). Vet Pathol. 2012;50(4):668-675.
7. Wang W, Song G, Lin J, et al. Study of the revisited, revised, and expanded Silva pattern system for Chinese endocervical adenocarcinoma patients. Hum Pathol. 2019;84:35-43.
8. Yokouchi Y, Imaoka M, Syama A, Sanbuissho A. Mixed germ cell tumor with embryonal carcinoma, choriocarcinoma and epitheloid trophoplastic tumor in the ovary of a Cynomolgus monkey. Toxicol Pathol. 2011;39(3):553-558.